Degradations of human immunoglobulins and hemoglobin by a 60 kDa cysteine proteinase of Trichomonas vaginalis
Article information
Abstract
The present study was undertaken to investigate the role of cysteine proteinase of Trichomonas vaginalis in escaping from host defense mechanism. A cysteine proteinase of T. vaginalis was purified by affinity chromatography and gel filtration. Optimum pH for the purified proteinase activity was 6.0. The proteinase was inhibited by cysteine and serine proteinase inhibitors such as E-64, NEM, IAA, leupeptin, TPCK and TLCK, and also by Hg2+, but not affected by serine-, metallo-, and aspartic proteinase inhibitors such as PMSF, EDTA and pepstatin A. However, it was activated by the cysteine proteinase activator, DTT. The molecular weight of a purified proteinase was 62 kDa on gel filtration and 60 kDa on SDS-PAGE. Interestingly, the purified proteinase was able to degrade serum IgA, secretory IgA, and serum IgG in time- and dose-dependent manners. In addition, the enzyme also degraded hemoglobin in a dose-dependent manner. These results suggest that the acidic cysteine proteinase of T. vaginalis may play a dual role for parasite survival in conferring escape from host humoral defense by degradation of immunoglobulins, and in supplying nutrients to parasites by degradation of hemoglobin.
INTRODUCTION
Proteinases of Trichomonas vaginalis participate in attachment to epithelial cells, pathogenic change of the host tissue, cytotoxicity and immune responses in host-parasite relationship (Alderete and Garza, 1988; Arroyo and Alderete, 1989). Trichomonas vaginalis secretes numerous proteinases during cultivation (Lockwood et al., 1987; North et al., 1990; Scott et al., 1995) and in vivo. Proteinases were detected in vaginal washes of patients infected with T. vaginalis and antibodies against proteinases existed both in sera and vaginal washes (Alderete et al., 1991; Bózner et al., 1992). On the other hand, excretory-secretory product (ESP) of T. vaginalis degrades human IgG, IgM, and IgA (Provenzano and Alderete, 1995). These findings suggested that proteinases of T. vaginalis may play an important role in immune evasion mechanism of the parasite. A purified proteinase of T. vaginalis ESP, an acidic proteinase, has a molecular weight of 60 kDa (Garber and Lemchuk-Favel, 1994). Recently, We reported that the characterization of a partially purified proteinase from T. vaginalis (Min et al., 1996). Live trophozoites, whole lysates, and T. vaginalis ESP degraded several kinds of immunoglobulins (Min et al., 1997), suggesting that T. vaginalis can evade host immune responses by digesting immunoglobulins. However, there still remains what types of proteinase are responsible for such phenomenon. Therefore, we purified a proteinase from T. vaginalis lysates, determined the enzyme family by various proteinase inhibitors, and characterized its biological properties such as the degradation of human immunoglobulins and hemoglobin.
MATERIALS AND METHODS
Cells and lysates
A KT9 isolate of T. vaginalis was obtained from a vaginal swab of a Korean women. Axenized parasites were cultured in a TYM medium and subcultured daily. Parasites were harvested at the log phase of the growth, sonicated in 0.1 M phosphate buffer (pH 7.0), and the soluble lysates were obtained by ultracentrifugation at 100,000 g for 1 hr at 4℃.
Assay of proteinase activity
Proteinase activity was determined by the method of Min et al. (1996). The reaction mixture containing 20 µl of the enzyme was preincubated with 40 µl of 10 mM dithiothreitol (DTT, Sigma, USA) for 5 min at room temperature and 20 µl of 1 mM N-benzoyl-prolyl-phenylalanyl-arginine-ρ-nitroanilide (Bz-Pro-Phe-Arg-Nan, Sigma) was added. Sodium phosphate buffer (0.1 M, pH 6.0) was added up to 400 µl and the reaction mixture was incubated for 1.5 hr at 37℃. Enzyme activity was measured at 405 nm using a spectrophotometer (Perkin Elmer, USA). One unit of enzyme activity was defined as an amount of enzyme which causes an increase of an absorbance change of 0.1 O.D./hr.
Purification of a proteinase from T. vaginalis lysate
1) Activated thiol-Sepharose 4B affinity chromatography
Lysates of T. vaginalis were applied to an activated Thiol-Sepharose 4B column (1.5 × 20 cm, Pharmacia, Sweden) pre-equilibrated with 0.1 M Tris-HCl (pH 7.5) containing 0.3 M NaCl and 1 mM EDTA. The bound proteins were eluted with the same buffer containing 30 mM DTT. The fraction with proteolytic activities were pooled, concentrated and dialysed.
2) Bacitracin-Sepharose 4B affinity chromatography
The active fractions of activated Thiol-Sepharose 4B chromatography were applied to a Bacitracin-Sepharose 4B column (1.0 × 10 cm, Pharmacia) pre-equilibrated with 20 mM sodium acetate buffer (pH 4.0). The bound fractions were eluted with 0.1 M Tris-HCl (pH 7.0) containing 1.0 M NaCl and 25% (v/v) propanol. The active fractions were pooled, concentrated and dialyzed.
3) Sephacryl S-200 HR gel filtration
To determine the molecular weight of a proteinase, active fractions of Bacitracin-Sepharose 4B chromatography were applied to a HiPrep Sephacryl S-200 HR gel filtration column (1.6 × 60 cm, Pharmacia) pre-equilibrated with 0.1 M sodium acetate (pH 5.5) containing 0.15 M NaCl. Proteins were eluted using the same buffer at a flow rate of 0.5 ml/min. The proteolytic fractions (1.5 ml each) were pooled, concentrated and dialyzed. The molecular weight was determined with proteins of known molecular weight (Pharmacia-LKB, Sweden) including bovine serum albumin (66 kDa), ovalbumin (43 kDa), chymotrypsinogen (25 kDa) and ribonuclease A (13.7 kDa).
Optimum pH of a purified proteinase
The optimum pH of the proteinase activity was determined by assaying the proteinase activity at pH 4.0, 4.5, 5.0 and 5.5 in 0.1 M sodium acetate, and at pH 6.0, 6.5, 7.0, 7.5 and 8.0 in 0.1 M sodium phosphate. Procedures for the proteolytic assay were done as described above using Bz-Pro-Phe-Arg-Nan as a substrate.
Effect of proteinase inhibitors
The purified proteinase was preincubated at 37℃ for 40 min with various inhibitors. The cysteine proteinase inhibitors used in this study were iodoacetic acid (IAA, 1 mM), N-ethylenemaleimide (NEM, 1 mM) and trans-epoxysuccinyl-L-leucylamido-(4-guanidino) butane (E-64, 0.1 mg/ml). An aspartic proteinase inhibitor (0.1 mg/ml pepstatin A), a metallo proteinase inhibitor (1 mM EDTA), a serine proteinase inhibitor (1 mM phenylmethyl sulfonyl fluoride (PMSF)), serine and cysteine proteinase inhibitors (1 mM N-β-tosyl-L-phenylethyl chloromethyl ketone (TPCK), 1 mM N-α-tosyl-L-lycine chloromethyl ketone (TLCK) and 0.1 mg/ml leupeptin) were also used. The effect of DTT, an activator of cysteine proteinase, and Hg2+ (HgCl2) were also assayed. All chemicals were purchased from Sigma except for E-64 which was purchased from Boehringer Mannheim, Germany.
Degradation of immunoglobulins
To observe the degradation of immunoglobulins, the purified proteinase (1, 2 or 5 µg) was incubated with 15 µg of serum IgG, serum IgA, or secretory IgA in the presence of 10 mM DTT at 37℃ for 1, 2, 5, 12, and 24 hr. The reaction was stopped by the addition of reducing sample buffer of SDS-PAGE. The degradation products of immunoglobulins were transferred to nitrocellulose paper, and immunoblot was undertaken using 1:1,000 dilution of peroxidase conjugated anti-human Fc IgG (Cappel, USA) or IgA (Cappel) as a secondary antibody.
Degradation of hemoglobin
Fifty µg of hemoglobin (Sigma) was incubated with 1, 2, 5, and 10 µg of the purified proteinase for 12 hr at 37℃ in the presence of 10 mM DTT. The reaction was stopped by the addition of reducing sample buffer of SD-SPAGE. The degradation products were analyzed by SDS-PAGE.
RESULTS
A proteinase of T. vaginalis was purified by activated thiol-Sepharose 4B, Bacitracin-Sepharose 4B, and Sephacryl S-200 HR gel filtration column in sequence. Molecular weight of the purified proteinase was estimated as 62 kDa by Sephacryl S-200 HR and 60 kDa by SDS-PAGE (Fig. 1).
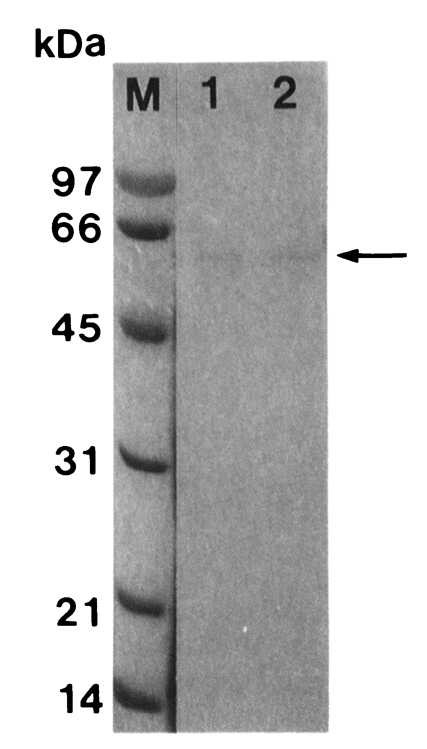
SDS-PAGE (12%) analysis of a cysteine proteinase (arrow) purified from crude extract of Trichomonas vaginalis according to sequential chromatographic steps. M, marker; lane 1, active peaks from Bacitracin-Sepharose 4B affinity chromatography; lane 2, active peaks from Sephacryl S-200 HR gel filtration.
The activity of the purified proteinase was approximately 78-fold higher than that of the crude extract, and the recovery was 0.4%. Maximum activity of this purified proteinase was observed at pH 6.0. The activity of purified proteinase was inhibited by cysteine proteinase inhibitors such as E-64, NEM, IAA and leupeptin, cysteine and serine proteinase inhibitors such as TPCK and TLCK, and Hg2+. On the other hand, this enzyme was activated by DTT. Other serine-, metallo-, and aspartic proteinase inhibitors such as PMSF, EDTA, and pepstatin A did not affect the activity of the enzyme (Table 1).
The purified proteinase degraded immunoglobulins in a dose- and time-dependent manner. After 1 hr of incubation, a degradation products of serum IgG, 35 kDa fragment was first observed, and heavy chain (50 kDa) was degraded into 32 and 27 kDa products after further reaction (Fig. 2).
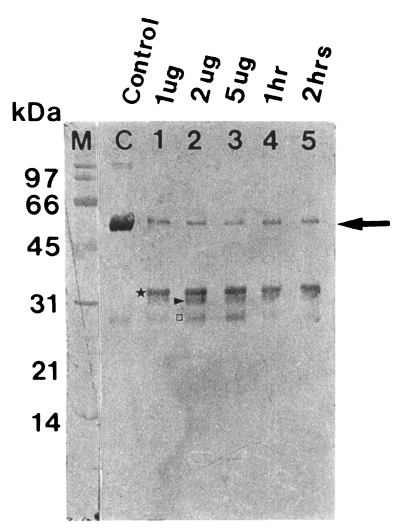
Degradation of human serum IgG by the purified 60 kDa cysteine proteinase. M, markers; C, serum IgG; lane 1-3, serum IgG incubated with 1, 2 and 5 µg enzyme for 5 hr; lane 4 & 5, serum IgG incubated with 2 µg enzyme for 1 and 2 hr. SDS-PAGE (12%) of IgG and immunoblot with goat anti-human Fc IgG were done. The cysteine proteinase digested heavy chain of serum IgG in a dose-dependent manner (lane 1-3) and a time-dependent manner (lane 4 & 5). Heavy chain (→) is degraded into 35 kDa (★), 32 kDa (▶) and 27 kDa (□).
When the degradation products of serum IgA were probed using sheep anti-human Fc IgA, heavy chain became gradually fainter according to the incubation time in a dose-dependent manner, leaving degradation products of 48, 45, and 35 kDa. After 24 hr of incubation, it was completely digested (Fig. 3A). A similar pattern of proteolysis was observed with secretory IgA, which was degraded into 48 kDa protein. Secretory IgA was more rapidly degraded compared to serum IgG and serum IgA (Fig. 3B).
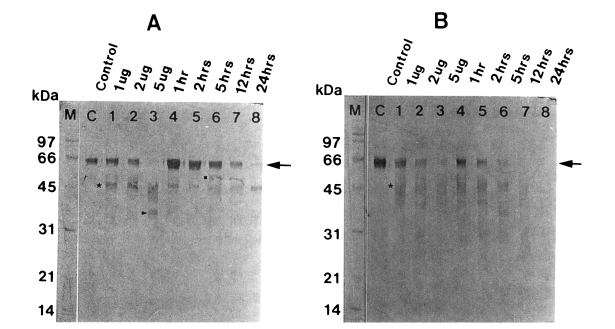
Degradation of human IgA (A) and secretory IgA (B) by the 60 kDa cysteine proteinase. M, markers; C, serum IgA (A) or secretory IgA (B); lane 1-3, serum IgA (A) or secretory IgA (B) incubated with 1, 2 and 5 µg enzyme for 5 hr; lane 4-8, serum IgA (A) or secretory IgA (B) incubated with 2 µg enzyme for 1, 2, 5, 12 and 24 hr. SDS-PAGE (12%) of IgA or secretory IgA and immunoblot with sheep anti-human Fc IgA were done. The cysteine proteinase digested heavy chain of serum IgA in a dose-dependent manner (lane 1-3) and a time-dependent manner (lane 4-8). Heavy chain of serum IgA (A) (→) is degraded into 48 kDa (■), 45 kDa (★) and 35 kDa (▶), and that of secretory IgA (B) (→) is degraded into 48 kDa (★).
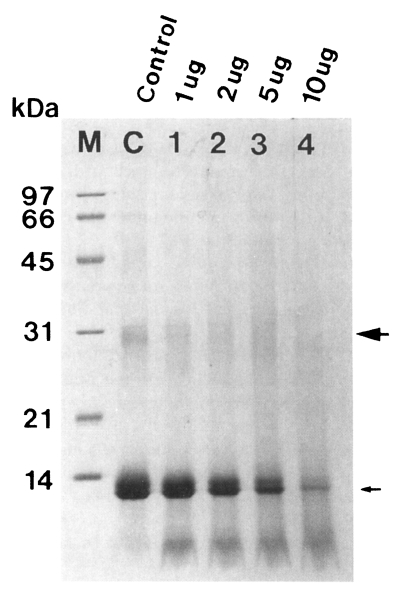
The purified enzyme also degraded dimer and monomer of hemoglobin in a dose-dependent manner, which were nearly degraded after 12 hr of incubation (Fig. 4).
DISCUSSION
Several investigators reported the degradation of immunoglobulins by live and lysates of T. vaginalis (Provenzano and Alderete, 1995; Min et al., 1997), and the relationships between host immune response and cysteine proteinase (Plaut, 1983; Parenti, 1989). We have partially purified a 60 kDa proteinase previously (Min et al., 1996) although it was mixed with other enzymes. In this study a proteinase from T. vaginalis lysates was purified using two affinity chromatography and gel filtration, and its biological properties such as the degradation of human immunoglobulins and hemoglobin were characterized.
The activity of this purified proteinase was maximal at pH 6.0. The enzyme activity was inhibited by E-64, IAA, NEM, leupeptin, TPCK, TLCK, and Hg2+, but not by PMSF, EDTA, and pepstatin A. These results suggest that the 60 kDa enzyme of T. vaginalis is an acidic cysteine proteinase, which is similar to those of other parasites such as Clonorchis sinensis, Schistosoma mansoni, Entamoeba histolytica, and Giardia lamblia (Chappell and Dresden, 1986; Parenti, 1989; Song et al., 1990; Avila and Calderon, 1993).
Min et al. (1994) demonstrated that the membrane protein with molecular weight of 60 kDa is one of the major protein of T. vaginalis. Previously, Garber and Lemchuk-Favel (1994) reported that a 43 kDa protease, a subunit of an extracellular protease at 60 kDa, was associated with cell surface of T. vaginalis. Interestingly, the cysteine proteinase purified from this study and the surface antigen have the same molecular weight of 60 kDa. The cellular localization of cysteine proteinase purified from the present study should be further evaluated by immunohistochemistry or immuno-electronmicroscopic method.
IgA provides a primary immunity to parasite and bacteria localized in the gastrointestinal tract, the lung, and the urogenital system (Janeway and Travers, 1994). Secretory IgA inhibits the mucous adherence of bacteria and parasites, and an import of pathogenic material into epithelial cells (Tagliabue et al., 1983). In vaginal washes of trichomoniasis patients, significantly elevated levels of IgA were found (Ackers et al., 1975; Su, 1982). To escape from host immune responses, some bacteria secrete proteinases to degrade host immunoglobulins. Protozoa such as G. lamblia, E. histolytica and T. vaginalis also secrete cysteine proteinases which degrade the host immunoglobulins (Plaut, 1983; Parenti, 1989; Kelsall and Ravdin, 1993; Provenzano and Alderete, 1995; Min et al., 1997). In this study, the purified cysteine proteinase degraded immunoglobulins, suggesting that T. vaginalis can escape from host immune surveillance by degrading IgA and secretory IgA. Because parasitic protozoans cause chronic disease in the host in general, protozoan cysteine proteinase may play a role in escaping from host humoral response.
Hemoglobin is used as a major nutrient by blood parasites such as Schistosoma mansoni and malaria, and is degraded by their proteinase into heme and globin, and the latter is subsequently degraded into free amino acids as a source of protein biosynthesis in parasites (Chappell and Dresden, 1986; Salas et al., 1995). The purified cysteine proteinase of T. vaginalis also degraded both the monomeric and dimeric forms of hemoglobin, implying that T. vaginalis may use the hemoglobin as a nutrient.
Iron is an essential nutrient to the trichomonads and potentially could be acquired from hemoglobin following hemolysis (Dailey et al., 1990). When hemoglobin as a sole source of iron is added to the growth medium under iron-limiting conditions, T. vaginalis showed excellent growth than the iron-chelated culture medium control (Alderete et al., 1992). In addition, iron mediates resistance of T. vaginalis to complement lysis and regulates growth of trophozoites and cytoadherence, which is associated with pathogenicity of T. vaginalis (Lehker et al., 1991; Lehker and Alderete, 1992; Alderete et al., 1995). Therefore, ability of degrading hemoglobin may be related with immune evasion and pathogenicity of T. vaginalis other than nutrient supply to trophozoites.
From this study, we hypothesized that a 60 kDa cysteine proteinase of T. vaginalis play roles not only in escape from host humoral response, but also in the supply of nutrients to parasite.
Notes
This study was supported by the grant from Ministry of Education for Basic Medical Science, 1996 (#96-284).