Biological and biochemical modulation of Trichomonas vaginalis KT9 isolate after shifting of culture medium from TPS-1 into TYM
Article information
Abstract
To evaluate the biological and biochemical characteristics of Trichomonas vaginalis KT9 isolate, the growth and size of trichomonads, pathogenicity in mouse, protein profiles and proteinase activity were examined after shifting the medium from TPS-1 into TYM. Generation time of trichomonads in TYM medium was 4.5 hr in comparison to TPS-1 with 7.1 hr. Size of trichomonads cultured in TPS-1 medium (8.5 ± 0.9 × 6.0 ± 0.9 µm) was significantly smaller than those in TYM medium (10.9 ± 1.4 × 8.2 ± 0.9 µm). Trichomonads cultured in TYM medium produced subcutaneous abscess in 9 out of 10 mice, whereas those in TPS-1 medium produced abscesses in 2 out of 10 mice. In SDS-PAGE, trichomonad lysates from both media showed ten common bands. However, trichomonads in TYM medium showed additional bands of 136 kDa, 116 kDa and 40 kDa in comparison to those in TPS-1 with 100 kDa. By immunoblot with T. vaginalis-immunized rabbit sera, T. vaginalis cultivated in both TYM and TPS-1 media showed 5 common bands, and unique bands of 116 kDa, 105 kDa, and 86 kDa were observed in trichomonads in TYM while a 140 kDa band in those in TPS-1. In gelatin SDS-PAGE, trichomonads in TYM degraded gelatin stronger than those in TPS-1. Also protease activity of trichomonads in TYM was significantly higher than that of trichomonads in TPS-1 using Bz-Pro-Phe-Arg-Nan as a substrate. According to the results, it is assumed that the shift from TPS-1 into TYM medium for cultivation of T. vaginalis might modulate the biological and biochemical properties of T. vaginalis in vitro.
INTRODUCTION
The growth condition has been known to affect the biological function and the pathogenicity of various protozoa such as Naegleria fowleri, Trichomonas vaginalis and Entamoeba histolytica. The proliferation of E. histolytica varies according to the batch of Panmede and bovine serum and components of TPS-1 culture medium (Chávez-Dueňnas et al., 1989). Trophozoites of N. fowleri grown in Cline medium and Nelson medium show differences in growth rate, locomotion rate, resistance to complement lysis, and the ability to destroy the nerve cell (Marciano-Cabral and Toney, 1994).
Several reports had shown that variation of pH or ingredients of media affects size and pathogenicity of T. vaginalis. Winston (1974) reported that variation in size of T. vaginalis was due to the effect of the host environment on a single species. Moon et al. (1983) and Kim et al. (1984) reported that trophozoites cultured under unfavorable pH conditions of TPS-1 medium were significantly larger than those cultured at an optimum pH (pH 7.0), and showed lower level of pathogenicity. Also, iron-sulfur protein including ferredoxin and pyruvate-ferredoxin oxidoreductase increased in T. vaginalis grown in iron-rich medium compared to that in low-iron medium (Gorrell, 1985).
We have cultivated T. vaginalis using TPS-1 medium for several years, originally devised for axenic cultivation of Entamoeba (Diamond, 1968). TYM medium is commonly used to cultivate T. vaginalis (Diamond, 1957). It was by mere chance that TPS-1 medium has been used for isolation of T. vaginalis instead of TYM medium in our laboratory. It was not known about alteration of T. vaginalis after changes of growth condition including shifting of media from TPS-1 to TYM. Therefore, this study was performed to observe the differences of biological and biochemical characteristics of T. vaginalis KT9 after medium change from TPS-1 into TYM medium.
MATERIALS AND METHODS
Trichomonas vaginalis and media
The KT9 isolate of T. vaginalis was obtained from a Korean woman with vaginitis (Ryu and Lloyd, 1995). Trophozoites were maintained in TPS-1 media for 3 years. For the change of media, trophozoites in TPS-1 media were transferred into TYM media (Table 1), and cultivated for about one year. Companies and catalogs numbers of the ingredients of TPS-1 and TYM media were as follows: BBL® Trypticase® pepton pancreatic digest of Casein (BD, USA), Panmede (Paines & Byrne limited, Greenford-England), yeast extract (Difco, 0123-17-3), maltose (Jassen chimica, B-2440, Geel Belgium), L-cysteine and ascorbic acid (Junsei Chemical Co.), Bovine serum (Hyclone), and Horse serum (Gibco BRL). Also D-glucose (G-8270), NaCl (S-7653), K2HPO4 (P-3786), KH2PO4 (P-5379), and FeSO4·7H2O (F-8263) were purchased from Sigma.
Iron concentrations of TYM and TPS-1 media measured by automatic chemical analyzer (Hidazi 747, Japan) were 2,200 µg/dl and 484 µg/dl, respectively.
Generation time and sizes of trophozoites
To measure generation time, trophozoites (3 × 104) at an early log phase were inoculated into 5 ml of TPS-1 and TYM media, respectively and the number of live trophozoites was quantitated using a hemocytometer twice a day.
For comparison of the size of trichomonads in each media, trophozoites were smeared on slide glasses and stained with Giemsa solution. The lengths and widths of each trophozoites were measured with measuring device attached at a personal computer (PC 8001, NEC). The Mann-Whitney U-test was used for comparison of data.
Animal assay
The 6-day subcutaneous lesion assaying method was employed in order to figure out the degrees of pathogenicity of T. vaginalis cultured in both media (Ryu et al., 1995). BALB/c mice were anesthesized with ether and T. vaginalis (1 × 106) from each media were inoculated into the subcutaneous tissue of the mice. After 1 week, mice were sacrificed and the size of abscesses were measured.
SDS-PAGE and immunoblot
SDS-PAGE and immunoblot were undertaken to observe the protein and antigenic profile of trichomonads. Trichomonas vaginalis in each media were sonicated and centrifuged at 10,000 g. Forty µg of the supernatant was loaded into the well. SDS-PAGE was carried out on a 7.5% running gel under discontinuous buffer system as the method of Laemmli (1970). Immunoblot was carried out according to the method of Tsang et al. (1983). Rabbit sera (1:500 diluted) immunized with T. vaginalis and peroxidase-conjugated anti-rabbit IgG (1:1000 diluted) were used for the reaction.
Gelatin SDS-PAGE
The ability of trophozoites to degrade gelatin was observed by gelatin SDS-PAGE in 7.5-15% gradient gel (Lockwood et al., 1987). After electrophoresis, gels were soaked in 2.5% Triton X-100 for 1 hr to remove SDS. Proteinase was activated by incubation for 4 hr in sodium phosphate buffer (pH 6.0) containing 1 mM dithiothreitol (DTT) at 37℃. The gels were stained in Coomassie brilliant blue R.
Proteinase activity
Proteinase activity was measured by modified methods of Lockwood et al. (1984). Fifteen µg of trichomonads supernatant was reacted with 15 µl DTT (10 mM) for 15 min, and 400 µl sodium phosphate buffer (pH 6.0) and 20 µl benzoyl-proline-phenylalanine-arginine-nitroanilide (Bz-Pro-Phe-Arg-Nan; 1 mM) were added to the reaction mixture. Enzyme activity was measured by optical density at 405 nm after incubation for 90 min at 37℃.
RESULTS
Generation time and sizes of trophozoites
The generation times of T. vaginalis cultivated in TYM and TPS-1 media were 4.5 hr and 7.1 hr, respectively (P=0.1). Growth of the trophozoites in TYM media did not show significant differences from that in TPS-1 (Fig. 1).
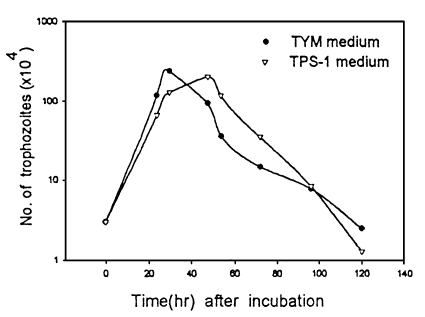
Propagation of Trichomonas vaginalis KT9 by inoculum dose (3 × 104) in TYM (•) and TPS-1 (▿) media. Trophozoites in TYM media grew faster than that in TPS-1, especially at an early log phase of growth.
Mean lengths and widths of trophozoites cultivated in TYM and TPS-1 media were 10.9 ± 1.4 × 8.2 ± 0.9 µm and 8.5 ± 0.9 × 6.0 ± 0.9 µl, respectively. Trichomonads of TYM media were significantly larger than those of TPS-1 media (P<0.0005).
Animal assay
Among 10 mice tested for pathogenicity of T. vaginalis, trophozoites in TYM media produced abscesses in 9 mice whereas trichomonads in TPS-1 produced abscess in 2 mice. The mean abscess size (11.7 ± 7.47 mm2) caused by trophozoites cultured in TYM was larger than that formed by trichomonads in TPS-1 (7.7 ± 0.55 mm2).
SDS-PAGE and immunoblot
In SDS-PAGE, common bands were observed at 75 kDa, 60 kDa, 58 kDa, 52 kDa, 47 kDa, 45 kDa, 43 kDa, 38 kDa, 37 kDa, and 32 kDa, and trichomonads in TYM and TPS-1 media showed additional bands at 136 kDa, 116 kDa and 40 kDa, and at 100 kDa, respectively (Fig. 2A). By immunoblot, T. vaginalis in both TYM and TPS-1 media showed 5 common bands at 95 kDa, 75 kDa, 60 kDa, 47 kDa, and 32 kDa, and trichomonads in TYM revealed unique bands at 116 kDa, 105 kDa and 86 kDa. By contrast, trophozoites in TPS-1 showed a band at 140 kDa (Fig. 2B).
Gelatin SDS-PAGE
Trichomonads in TYM media degraded gelatin stronger than those in TPS-1 media. Ten bands were observed in common and a band at 52 kDa was detected in trichomonads cultured in TPS-1 media only (Fig. 2C).
Proteinase activity
Enzyme activity using Bz-Pro-Phe-Arg-Nan was measured by optical density at 405 nm. Optical density (0.27 ± 0.07) of trichomonads in TYM media was significantly higher than that of trichomonads (0.11 ± 0.03) in TPS-1 (P < 0.01).
DISCUSSION
It has been known that small size of T. vaginalis might be related with stronger pathogenicity or more severe symptoms than large trophozoites (Winston, 1974; Moon et al., 1983; Kim et al., 1984). On the contrary, trichomonads in TYM media showed more stronger pathogenicity and larger size than those in TPS-1 in the present study.
Shim et al. (1993) reported that neutral and acid proteinase activities in pathogenic group of T. vaginalis were significantly higher than those of weak pathogenic group. In the present study, trichomonads in TYM showed higher proteinase activity and stronger pathogenicity than those in TPS-1. Therefore, it is supposed that the pathogenicity of T. vaginalis could be related with proteinase activity rather than the size of trophozoites.
The importance of iron for growth of T. vaginalis was demonstrated by several investigators (Gorrell, 1985; Lehker et al., 1991; Lehker and Alderete, 1992). Iron-sulfur protein for energy production required for survival of trichomonads increased in T. vaginalis grown in iron-rich medium compared with that in low-iron medium (Gorrell, 1985). Also surface expression and synthesis of adhesins, which is related with adhesion of trichomonads to epithelial cell, was mediated by iron concentration (Lehker et al., 1991). In the present study, iron concentrations of TYM and TPS-1 media were 2,200 µg/dl and 484 µg/dl, respectively. Although iron concentration of TPS-1 medium was lower than that of TYM, this value seems to be enough for T. vaginalis because trichomonads cultivated at a concentration of about 200 µg/dl of iron manifested strong pathogenicity (data not shown). Nonetheless it is desirable to compare TYM with TPS-1 supplemented with iron to confirm importance of iron concen-tration for cultivation of T. vaginalis.
TPS-1 medium was originally devised for axenic cultivation of Entamoeba (Diamond, 1968). Major ingredient of TPS-1 medium is Panmede, which is manufactured by enzymatic hydrolysis of bovine liver homogenates (Diamond, 1968). Although not precisely quantified, Panmede's quality is known to vary, and most lots cannot support amoebal growth (Diamond et al., 1978). Chávez-Dueňnas et al. (1989) reported that it can improve the growth of E. histolytica by selecting appropriate lots of Panmede.
Even though it is suggested that the difference of medium component, such as serum, Panmede, yeast extract and ferrous sulfate, between TYM and TPS-1 might affect the various pathophysiologic effects of T. vaginalis, it is difficult to find out which one or complex of medium components cause those differences.
Trophozoites in TYM media showed shorter generation time, larger size, stronger proteinase activity and virulence in comparison with those of TPS-1 media in the present study. Therefore, TYM media may be better than TPS-1 media for maintenance of various characteristics related with patho-genicity of T. vaginalis.
ACKNOWLEDGEMENTS
The authors would like to thank Mr. Han-Gyoo Choi, Department of Parasitology, College of Medicine, Hanyang University for his technical help during the study.
Notes
This study was supported by the Research Grant of the Faculty Research Project, Hanyang University, 1997

