Serosurveillance of Scrub Typhus in Small Mammals Collected from Military Training Sites near the DMZ, Northern Gyeonggi-do, Korea, and Analysis of the Relative Abundance of Chiggers from Mammals Examined
Article information
Abstract
Comprehensive quarterly serosurveillance on scrub typhus in small mammals collected from military training sites located near the Demilitarized Zone (DMZ), northern Gyeonggi-do (Province), ROK was conducted to determine the potential rodent-borne and associated ectoparasite disease risks to military personnel. A total of 1,196 rodents and insectivores representing 8 species, Apodemus agrarius (87.3%, n = 1,044), Mus musculus (5.4%, n = 65), Crocidura lasiura (3.3%, n = 40), Microtus fortis (2.6%, n = 31), Micromys minutus (0.3%, n = 4), Tscherskia triton (0.3%, n = 4), Rattus norvegicus (0.3%, n = 4), and Myodes regulus (0.3%, n = 4) were assayed for the presence of antibodies to Orientia tsutsugamushi. O. tsutsugamushi antibodies were detected in 6 of 8 species and seroprevalence determined; A. agrarius (45.6%), M. musculus (23.1%), M. fortis (48.4%), M. minutus (50.0%), T. triton (50.0%), and R. norvegicus (25.0%). A total of 31,184 chigger mites collected from 508 rodents and insectivores were slide-mounted and 10 species belonging to 4 genera were identified. Leptotrombidium pallidum (53.4%) was the most frequently collected, followed by L. palpale (15.7%), Neotrombicula tamiyai (14.3%), L. orientale (10.7%), L. zetum (3.1%), Walchia fragilis (2.1%), and L. gemiticulum (0.8%), while the remaining 3 species, L. subintermedium, N. gardellai, and Euschoengastia koreaensis were rarely observed (prevalence < 10%). In contrast to previous surveys, higher chigger indices of the primary scrub typhus vectors, L. pallidum (165.4), L. orientale (45.0), and L. palpale (21.4), were observed during the spring season.
INTRODUCTION
Orientia tsutsugamushi, the causative agent of scrub typhus, is transmitted by larval chigger mites and is widely distributed throughout the Orient, parts of the Palearctic, and the Australasian zoogeographical regions. Eight scrub typhus cases were first reported from 1951-1953 in UN army soldiers in the Republic of Korea (ROK) [1,2]. Later, 64 cases were reported in 1985 [3-5] and from 1998 through 2003, the number of cases reported among ROK civilian and military personnel increased to 1,140-2,638 cases. The numbers of cases increased steadily, and by 2008 more than 6,000 cases were reported [6]. Peak numbers of scrub typhus cases were reported from October through December in the ROK [6,7]. While there have been few confirmed scrub typhus cases (1 each year for 1995 and 2003) among US soldiers, it remains a serious health threat, as it can rapidly incapacitate large numbers of persons and degrade military operations. Therefore, it is important to identify the spatial and temporal distribution of vectors and associated pathogens to develop and institute disease mitigation strategies.
A total of 44 species of chigger mites have been reported from the ROK. Leptotrombidium pallidum and L. scutellare are the primary vectors of scrub typhus in the ROK. L. pallidum has the most widespread distribution, while L. scutellare is largely restricted to the southern half of the Korean peninsula and islands [8-17].
In the present study, small mammals collected at US and ROK operated military training sites were assayed for O. tsutsugamushi-specific antibodies to determine the prevalence of infection. In addition, chiggers were removed and identified to species to determine small mammal host associations and their relative seasonal abundance. This study was conducted to provide a more accurate assessment of the potential health risk of soldiers acquiring scrub typhus while conducting military training exercises near the DMZ, northern Gyeonggi Province, ROK.
MATERIALS AND METHODS
Survey areas and collection of rodents
Seasonal small mammal surveillance was conducted during the March, June, August, and November-December at 8 US and ROK operated military training sites near the DMZ, northern Gyeonggi-do (Province), ROK (Fig. 1). Small mammals were live captured utilizing Sherman® collapsible traps (7.7 × 9 × 23 cm; H.B. Sherman, Tallahassee, Florida, USA) baited with peanut butter placed between saltine crackers set in trap lines (25 traps) at approximately 4 m intervals before sunset and collected from 07:00-10:00 hrs the following morning as described by O'Guinn et al. [18]. Small mammals were transported to the College of Medicine, Korea University, where they were anesthetized; euthanized by exsanguination by cardiac puncture under an approved animal use protocol (Korea University), identified, sexed, weighed, and tissues (spleen, lung, and kidney) removed and stored at -70℃ until used.
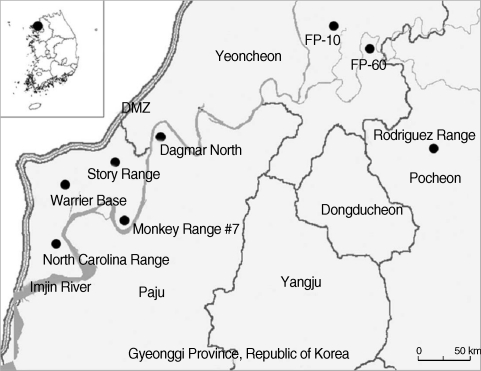
Map of small mammal collection sites at US and ROK operated training sites near the Demilitarized Zone (DMZ), northern Gyeonggi Province, Republic of Korea [North Carolina Range: Jangdan-myeon, Paju-si, Gyeonggi Province (37° 53'31.11''N, 126° 43'20.22''E); Warrior Base: Gunnae-myeon, Paju-si, Gyeonggi Province (37° 55'15.02''N, 126° 44'45.04''E); Monkey Range #7: Jindong-myeon, Paju-si, Gyeonggi Province (37° 53'44.30''N, 126° 48'08.61''E); Story Range: Jindong-myeon, Paju-si, Gyeonggi Province (37° 57'14.85''N, 126° 48'17.12''E); Dagmar North: Jeokseong-myeon, Paju-si, Gyeonggi Province (37° 58'02.32''N, 126° 50'27.00''E); Firing Point 10: Yeoncheon-gun, Gyeonggi Province (38° 04'41.70''N, 127° 04'37.00''E); Firing Point 60: Yeoncheon-gun, Gyeonggi Province (38° 02'55.34''N, 127° 06'18.18''E); Rodriguez Range: Youngjung-myeon, Pocheon-gun, Gyeonggi Province (38° 01'04.29''N, 126° 43'10.12''E)].
Orientia tsutsugamushi-specific antibody detection
Blood samples were centrifuged at 1,000 g for 10 min, and sera separated and maintained at -70℃ until assayed for the presence of O. tsutsugamushi-specific antibodies. A total of 30 µl of sera from each small mammal was diluted 1 : 64 in PBS and examined for IgG antibodies against O. tsutsugamushi Karp and Gilliam strains by the indirect immunofluorescence assay (IFA) technique. The IFA antigen slide was placed in a moist chamber to maintain humidity throughout the procedure. Diluted sera to be tested were deposited on a spot slide, incubated at 37℃ for 30 min, and then washed with 3 changes each for 5 min with PBS (10 mM, pH 7.2). Fluorescein isothiocyanate-conjugated goat anti-mouse or rat antibody (MP Biomedicals, Aurora, Ohio, USA) (30 µl), was pipetted onto each spot, and the slides were then incubated in a humidified chamber at 37℃ for 30 min. The slides were washed 3 times each for 5 min with PBS and then air-dried. The slide spots were mounted with glycine-buffered glycerol under cover slips and examined for characteristic cytoplasmic fluorescent patterns with a fluorescence microscope (50 W, Zeiss Co, Mainz, Germany).
Collection of chigger mites
Chigger mites were removed from the ears of euthanized small mammals using fine forceps under a dissecting microscope, placed in 80% ethanol, subsequently mounted on glass slides in Hoyer's mounting media, and then identified to species at × 400 using a standard key for chigger mites in Korea [19].
RESULTS
O. tsutsugamushi-specific antibodies in small mammals
A total of 1,196 rodents and soricomorphs (insectivores) representing 8 species and 8 genera were collected (Table 1). Apodemus agrarius (87.3%) was the most frequently collected small mammal, followed by Mus musculus (5.4%), Crocidura lasiura (3.3%), Microtus fortis (2.6%), Micromys minutus (0.3%), Tscherskia triton (0.3%), Rattus norvegicus (0.3%), and Myodes regulus (0.3%). Antibodies reactive to O. tsutsugamushi Karp, Kato, and Gillian strain antigen preparations were detected in 6 of 8 small mammal species; M. minutus (50.0%), T. triton (50.0%), M. fortis (48.4%), A. agrarius (45.6%), M. musculus (23.1%), and R. norvegicus (25.0%) (Table 2). A. agrarius seropositive rates for all trapping periods ranged from 26.9% to 58.3%. High seropositive rates for collection sites were recorded for Firing Point 10 (FP-10) (Yeoncheon, 82.1%), followed by Warrior Base (Paju, 70.8%) and Monkey Range #7 (Paju, 69.9%), whereas seropositive rates at other military training sites were relatively low (range 10.3-35.2%). The highest seropositive rate (94.3%) was recorded at FP-10 during the spring season, while low seroprevalence rates were observed for all training sites surveyed during August (Table 3).

Rodent and insectivore species diversity at selected US and ROK operated military training sites, northern Gyeonggi Province, Republic of Korea, 2003

Seropositive rates of scrub typhus (Orientia tsutsugamushi) in small mammals collected at military training sites, northern Gyeonggi province, Republic of Korea, 2003
Collection of chigger mites
A total of 31,184 chigger mites belonging to 10 species and 4 genera were collected from 508 rodents and soricomorphs (42.5% of total collected small mammals). Rattus norvegicus (325.0) had the highest chigger index (number of larval mites/small mammal) for L. pallidum, a scrub typhus vector, followed by M. fortis (136.5), M. minutus (51.0), A. agrarius (31.9), M. musculus (7.2) and C. lasiura (0.7) (Table 4). Overall, L. pallidum was the most commonly collected (53.4%), followed by L. palpale (15.7%), Neotrombicula tamiyai (14.3%), L. orientale (10.7%), L. zetum (3.1%), Walchia fragilis (2.1%), and L. gemiticulum (0.8%). The remaining species, L. subintermedium, N. gardellai, and Euschoengastia koreaensis were only collected from A. agrarius, with indices < 0.1 (Table 4). Overall, 4 vectors of scrub typhus (L. pallidum, L. palpale, L. orientale, and L. zetum) accounted for 82.9% of all chiggers collected from all rodents and soricomorphs, whereas non-vector species (N. tamiyai and W. fragilis) accounted for 14.3% and 2.1%, respectively (Table 4). Neotrombicula tamiyai was the most commonly collected non-vector chigger mite from A. agrarius, M. fortis, M. minutus and C. lasiura, while W. fragilis was the most commonly collected non-vector mite from M. fortis and T. triton (Table 4). High chigger indices of L. pallidum (145.3), L. orientale (33.9), and L. palpale (22.8) were recorded from all small mammals during the spring (March) compared to the other seasons (Table 5). In contrast, N. tamiyai was collected more frequently during the winter (November-December), while W. fragilis was collected more frequently during the early summer (June) and late summer (August) (Table 5).

Total number of the chigger mites (chigger indices), by species and host, captured at military training sites near the demilitarized zone, northern Gyeonggi Province, Republic of Korea, 2003

Seasonal chigger indices, by species, collected from rodents and insectivores captured at military training sites near the demilitarized zone, northern Gyeonggi Province, Republic of Korea, 2003
A total of 24,628 mites were collected from 448 A. agrarius. Four scrub typhus vectors (L. pallidum, L. palpale, L. orientale, and L. zetum) and 1 non-vector species (N. tamiyai) accounted for 84.3% and 14.7%, respectively, of all chiggers (Table 4). Significantly higher vector chigger indices of L. pallidum (165.4), L. orientale (45.0), and L. palpale (21.4) were recorded from A. agrarius during the spring season compared to N. tamiyai, which was collected more frequently during the winter (Table 6).
DISCUSSION
A comprehensive seasonal rodent-borne disease surveillance program, which included serosurveillance for evidence of O. tsutsugamushi (the causative agent of scrub typhus) infection in small mammals and identification of larval chigger mites, was conducted in 2003 at 8 US and ROK operated training sites located near the DMZ. While few cases of scrub typhus have been reported in US soldiers over the last decade, a pre- and post-deployment serosurvey determined that 0.2% (15/9,135) seroconverted to scrub typhus while deployed to Korea in 1995 [20]. Reasons for these undiagnosed cases are unknown. However, evidence suggests that serological techniques (i.e., ELISA) used for diagnosis are often not positive for up to 30 days after infection and follow-up characterization of illnesses are infrequently done once the soldier is released and returned to duty [18,21]. Overall, the observed prevalence of O. tsutsugamushi specific antibodies were moderately high in A. agrarius (45.6%) and were similar to other survey results where US military train (Dagmar North, seasonal range 25-38%; Firing Points 10 and 60, 48-72%; and Twin Bridges, 47-60%) and other non-military sites throughout the ROK (range 0-81.1%) [22-24]. Similar to Ree et al. [11], we observed evidence of previous or current infections of O. tsutsugamushi in A. agrarius, R. rattus, M. minutus, and M. musculus. Crocidura lasiura, collected from grassy habitats where infected A. agrarius were commonly collected, were infrequently seropositive for O. tsutsugamushi-specific antibodies and was likely related to observed low vector species indices [9-11,13,18,25].
There are 44 species of chigger mites, with L. pallidum, L. scutellare, L. palpale, L. orientale, L. zetum, and E. koreaensis implicated as vectors of scrub typhus, in the ROK [8,12,25-27]. Similar to other studies, L. pallidum was the predominant chigger taken from A. agrarius in areas near the DMZ [8,14-16,26]. Unlike Ree et al. [28], and Lee et al. [15] who observed high chigger indices of L. pallidum (range 43.6-136.1) on A. agrarius in the fall (October) at Gyeonggi-do (Province), our results showed the highest indices (165.4) during the spring (March) period, with relatively low indices during June, August, and November-December (14.2, 8.4, and 28.4, respectively). These data do not conform to the high incidence of scrub typhus from late September-early December observed in the Korean population, which may in part be related to increased human exposure during harvesting and other outdoor activities. Additionally, small mammal surveillance was conducted prior to the onset of the primary "scrub typhus transmission season", and therefore may account for the low larval chigger indices from A. agrarius and other small mammals. While the number of scrub typhus cases diagnosed in the Korean population greatly increased from 2,638 in 2001 to 6,708 in 2005, the annual seroprevalence in A. agrarius decreased from 79% (2002) to 49% (2005) at FP-10 and 60, while remaining relatively stable at Dagmar North (range 27-36%) [22,24]. Therefore, other factors, e.g., pharmacies not issuing prescription drugs without a doctor's request, improved diagnostics, adoption of a 40 hr workweek, which allows more time for outdoor hiking and picnics, and increased awareness, must be considered.
Surveillance of small mammals and associated zoonotic diseases was conducted in military-restricted areas near the DMZ where ROK and US soldiers are deployed and/or train. The presence of vectors of O. tsutsugamushi, relatively high seroprevalence rates among rodents, and transitory vegetation in disturbed environments, all of which are characteristic of military training sites located near the DMZ, place soldiers training in these habitats at risk for scrub typhus. Cantonment sites are often established adjacent to tall grasses and forested margins, while training activities place them in chigger infested habitats along roadsides, firing positions, or other grassy areas. Soldiers that properly wear their uniform (pant legs tucked into their boots) and the increased use permethrin-treated uniforms greatly decrease risk of larval chigger bites and may account for the low proportion (0.2%) of soldiers that seroconverted to O. tsutsugamushi during the 1995 pre- and post-serosurveillance of > 9,000 US soldiers deployed to the ROK [10]. Evaluations of environmental modifications (e.g., cutting vegetation to < 10 cm) are necessary to determine its effects on decreasing small mammal and chigger populations and the potential for disease transmission where soldiers conduct field training [29,30].
Finally, surveillance of small mammal and their ectoparasites, such as this, can be used to produce ecologic niche models that provide information on the spatial and temporal patterns of rodent populations and potential for disease transmission and provides necessary baseline data that can be used for developing, instituting, and evaluating the success of disease mitigation strategies.
ACKNOWLEDGEMENTS
We thank members of 5th and 38th Medical Detachment for conducting small mammal collections as part of the rodent-borne disease surveillance program of 65th Medical Brigade. We also appreciate the support from Dr. Glen Livet that he provided during this study. Funding for the portion of this work was provided by the Armed Forces Health Surveillance Center, Global Emerging Infections Surveillance and Response System, Silver Spring, MD, and the National Center for Medical Intelligence, Ft Detrick, MD.
Disclaimer
The views expressed in this article are those of the authors and do not reflect the official policy or position of the Department of Defense, the Department of the Army, or the US Government.

