The hard tick
Dermacentor silvarum Olenev, one of the principal vectors of Russian spring-summer encephalitis virus,
Babesia equi,
Babesia caballi,
Rickettsia slovaca, and
Rickettsia raoultii, is widely distributed in northern China, Russia, and Mongolia, and has an impact on human health as well as economic losses [
1,
2]. Previous studies on
D. silvarum mainly focused on its life cycle and biological characteristics [
3], and no candidate vaccines against biting
D. silvarum have been reported. Thus, searching for a protective antigen as an effective vaccine against
D. silvarum infection is necessary.
Tick subolesin (4D8) is the ortholog of insect akirins, which are evolutionarily conserved proteins that affect the innate immune response, reproduction, and development of ticks [
4-
6]. Vaccination trials with the recombinant 4D8/akirins antigens reduced fertility and survival, not only in ticks, but also in mosquitoes and sand flies [
7]. Here, the
D. silvarum 4D8 (Ds4D8) gene was cloned, and immunogenicity of the recombinant Ds4D8 (rDs4D8) was analyzed by western blot.
Adult
D. silvarum were collected from infested sheep at the Xiaowutai National Natural Conservation Area, Hebei province, China and reared as described previously [
3]. To clone the Ds4D8 gene, 2 µg of total RNA was extracted from adult females using RNA purification kit (Axygen, Union City, California, USA) and reverse-transcribed to synthesize cDNA templates using a cDNA synthesis kit (ThermoScript RT-PCR system, Invitrogen, Carlsbad, California, USA). The forward primer FP-RA4D85 and reverse primer RP-RA4D83 (
Table 1) were used to clone the open reading frame (ORF) of Ds4D8 cDNA (
Ds4D8). PCR samples were amplified at 94℃ for 2 min, followed by 30 cycles of 94℃ for 30 sec, 59℃ for 30 sec, 68℃ for 1 min, and a final extension at 72℃ for 10 min. Subsequently, Ds4D8 protein sequences were aligned using ClustalX, and the output of a graphic file was used with DNAMAN software. Phylogenetic analysis was constructed using MEGA version 3.1. A bootstrap resampling analysis with 1,000 replicates was performed to evaluate the inferred tree topology.
The gene-specific primers with
EcoRI/XhoI restriction enzyme sites, the forward primer FP-EcoRI-RA4D85 and reverse primer RP-XhoI-RA4D83 (
Table 1), were designed to clone the
Ds4D8. PCR amplification was performed at 94℃ for 2 min, followed by 35 cycles of 94℃ for 30 sec, 59℃ for 30 sec, 72℃ for 50 sec, and a final extension for 10 min at 72℃. The PCR products were ligated into the pMD19-T cloning vector (Takara, Ohtsu, Japan), digested with restriction enzymes, and ligated into the pET-32 (a+) expression vector (Takara) to form the recombinant plasmid pET-32(a+)-4D8 which was transformed into
Escherichia coli rosetta. The recombinant protein rDs4D8 was fused with 22 kDa histidine-tagged thioredoxin protein (TRX), following the induction by 1 mM isopropyl β-D-thiogalactopyranoside (IPTG) for 5 hr at 37℃.
E. coli liquids of different treatment were centrifugated by 10,000 rpm for 10 min at 4℃ and the precipitate was broken up by ultrasonic disruption (power, 400 W), and 20 µg protein samples per lane were separated by 10% SDS-PAGE.
The immunogenicity analysis of rDs4D8 was performed by western blot. A total of 20 µg protein sample per lane, including prestained protein marker (Fermentas, Ontario, Canada), purified rDs4D8, and IPTG-induced E. coli containing pET-32(a+)-4D8, were separated by 10% SDS-PAGE and transferred to polyvinylidene difluoride (PVDF) membrane. The membrane was cut into 2 strips and blocked for 2 hr in PBS-Tween-20 (PBST) containing 5% fat-free milk, and then incubated with rabbit anti-D. silvarum serum (1:2,000) and rabbit negative serum overnight at room temperature, respectively. Following overnight incubation, they were washed with PBST and incubated with diluted peroxidase-conjugated affinipure goat anti-rabbit IgG (1:2,000) for 1.5 hr (Proteintech, Chicago, Illinois, USA). Positive signals were detected using supersignal® west dura extended duration substrate (Thermo Scientific, Rockford, Illinois, USA).
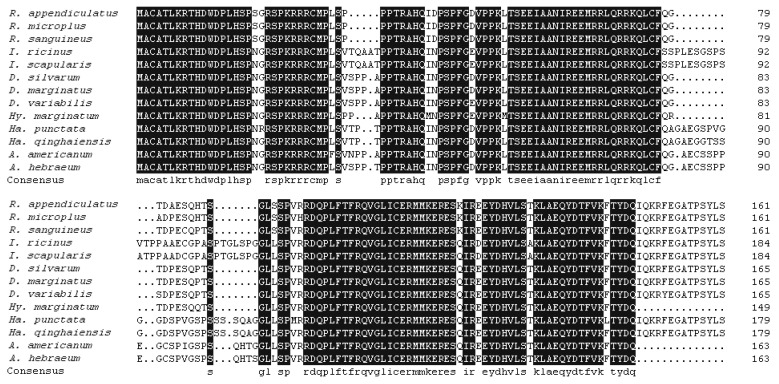
The
Ds4D8 sequence, consisted of 498 bp and encoding 165 amino acid residues, was submitted to GenBank (no. JX856138) (
Fig. 1). The predicted amino acid sequence was compared with ORF from 12 tick species belonging to 6 different genera (
Fig. 2). The results showed that Ds4D8 shared 100% identity with
Dermacentor marginatus, 98.2% identity with
Dermacentor variabilis, 95.4% identity with
Hyalomma marginatum, 93.9% identity with
Rhipicephalus sanguineus, 93.0% identity with
Rhipicephalus appendiculatus and
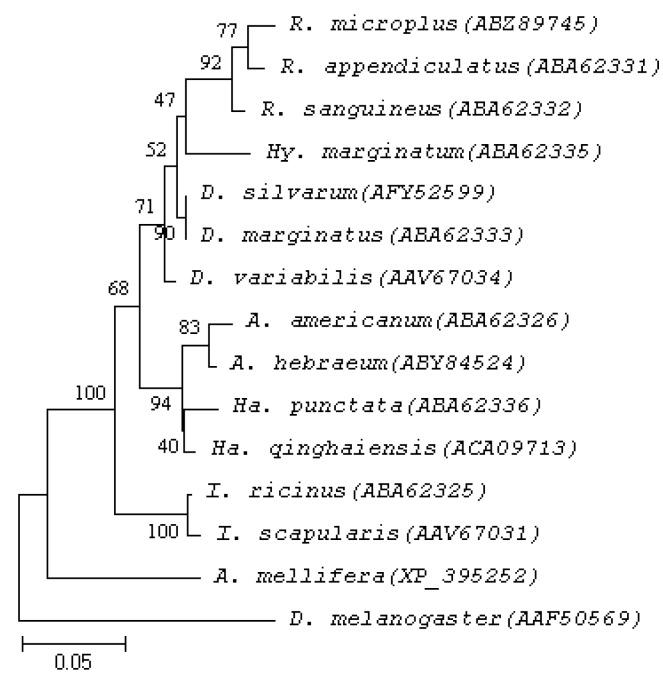
Rhipicephalus microplus, and 81.0-90.2% identity for ticks in the other 3 genera. In addition, the phylogenetic tree showed 2 major monophyletic clades (excluding outgroup sequences) and sequences clustered in each clade were classified as a subfamily (
Fig. 3). One monophyletic clade for 4D8 included
Ixodes ricinus and
Ixodes scapularis as a subfamily with bootstrap scores 100. The other monophyletic clade included
Rhipicephalus,
Dermacentor,
Hyalomma,
Haemaphysalis, and
Amblyomma spp.
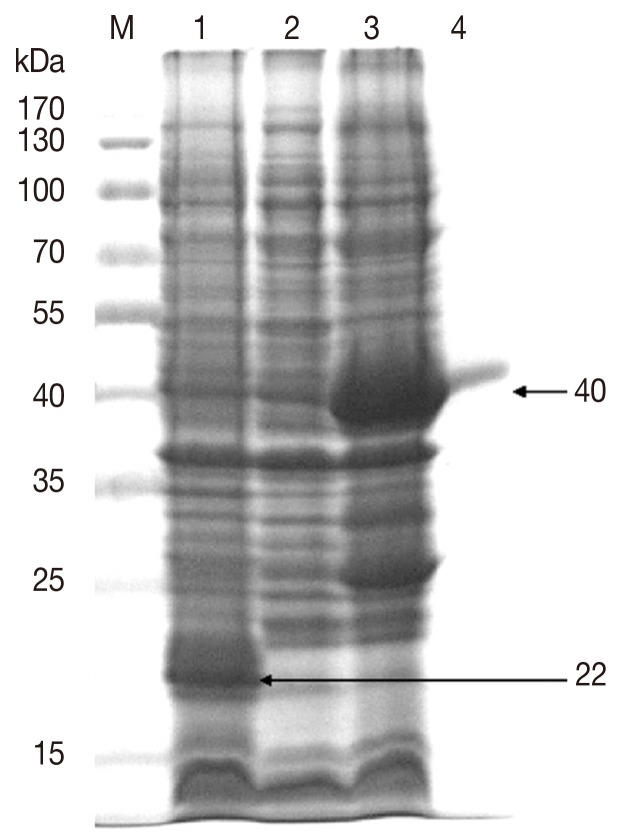
As shown in 10% SDS-PAGE gel (
Fig. 4), the rDs4D8 expression was induced by IPTG and its molecular weight was about 40 kDa, which was consistent with the expected molecular mass considering that the expression vector produced a recombinant protein fused with a 22 kDa TRX protein (
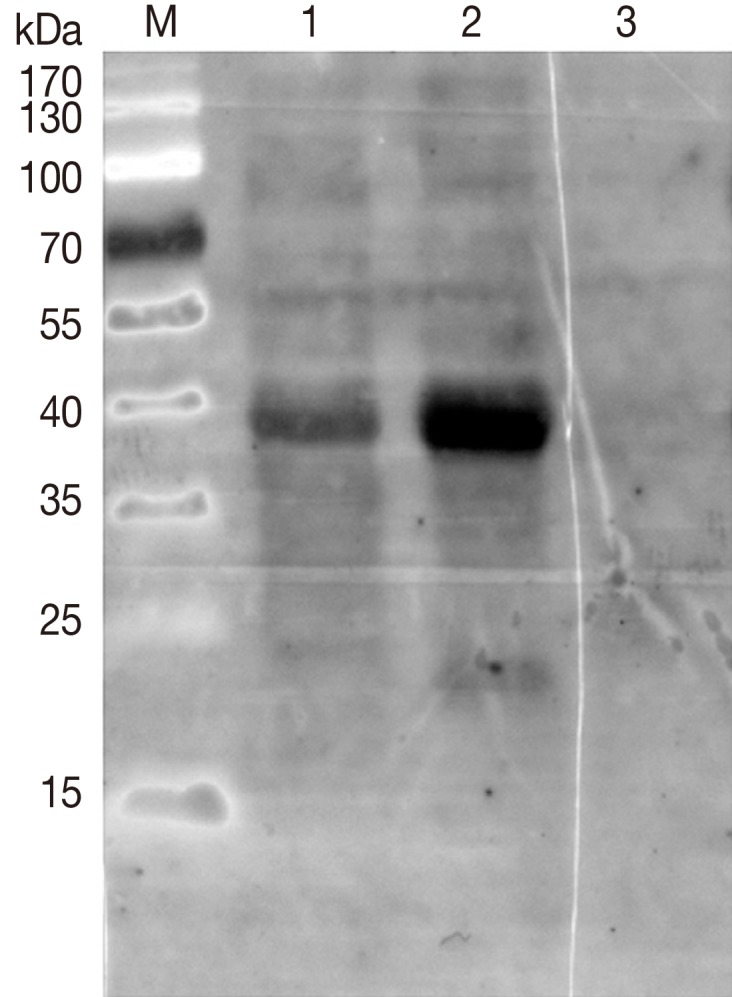
Fig. 4). The reactivities of the purified rDs4D8 and IPTG-induced
E. coli containing pET-32(a+)-4D8 with rabbit anti-
D. silvarum serum were tested by western blot. The results showed that the rDs4D8 (40 kDa) in lane 1 and lane 2 could be recognized by rabbit anti-
D. silvarum serum, while rabbit negative serum did not react with the rDs4D8 expressed in
E. coli of lane 3 (
Fig. 5).
The 4D8 plays a potential role in tick control. For example, de la Fuente et al. [
4] verified that the silencing of 4D8 expression after RNAi resulted in the reduction of tick weight, oviposition, and survival. Oviposition was reduced in over 90% of all tick species evaluated. Vaccination with recombinant tick 4D8 may control
R. microplus tick infestations in white-tailed deer to reduce tick populations, where the overall vaccine efficacy was 83% [
8]. In addition, Merino et al. [
9] confirmed that 4D8 vaccines also reduced the infection levels by 2 of the most important pathogens transmitted by cattle ticks,
Anaplasma marginale and
Babesia bigemina. To date, no 4D8 orthologs have been fully characterized nor tested as recombinant antigens in
D. silvarum.
In this study, we cloned
Ds4D8 containing 498 bp coding for 165 amino acids with a predicted molecular weight of 18 kDa, which was similar to the values observed for most 4D8 proteins [
4]. The results of multiple alignment and phylogenetic relationship demonstrated that 4D8 protein sequences are highly conserved among
Dermacentor,
Rhipicephalus, and
Hyalomma.
The 4D8 cDNA from
Ornithodoros erraticus was subcloned into the expression vector pQE-30 and expressed in
E. coli M15 cells [
10]. The western blot results showed that the molecular weight of
O. erraticus recombinant 4D8 was about 23 kDa containing 6×His-tagged recombinant polypeptides, while rDs4D8 had a larger molecular weight of 40 kDa when fused with pET-32(a+) tag protein. This may be due to the difference of expression vectors. In addition, Bensaci et al. [
11] cloned
I. scapularis 4D8 into vaccinia virus vector, and it was expressed from mammalian cells infected with the recombinant virus. The results suggested that immunized mice with
I. scapularis 4D8 by oral gavage inhibited tick feeding and transmission of
Borrelia burgdorferi. This provided a new way to analyze the function of rDs4D8 in
D. silvarum.
By combining the results of peptide and phage-display libraries scan analysis, tick 4D8 linear B-cell epitopes (T1, T2, and T3) were identified [
12]. Subsequently, the Q38 and Q41 chimeras were designed by combining 4D8 and akirin protective epitopes [
12]. Studies of immunized mice showed that tick molting from larvae to nymphs was significantly reduced by 14-48% when compared to controls [
13]. Our results of western blot showed that rDs4D8 demonstrated immunities against rabbit anti-
D. silvarum serum. These data provided a basis for future studies on immunization with rDs4D8 or 4D8 epitopes to prevent attachment of
D. silvarum ticks on target vertebrate hosts.
A Project of Education Department of Hebei Province2011160
Natural Science Foundation of Hebei ProvinceC2011205104
The Foundation of Hebei Normal University, ChinaL2012Z06L2008B10
ACKNOWLEDGMENTS
This work was supported by a Project of Education Department of Hebei Province (grant no. 2011160), Natural Science Foundation of Hebei Province (grant no. C2011205104) and the Foundation of Hebei Normal University, China (no. L2012Z06 and no. L2008B10).
CONFLICTS OF INTEREST
We declare that we have no conflict of interest related with this work.
REFERENCES
1. Yu ZJ, Zheng HY, Chen Z, Zheng B, Ma H, Liu JZ. The life cycle and biological characteristics of
Dermacentor silvarum Olenev (Acari: Ixodidae) under field conditions. Vet Parasitol 2010;168:323-328. PMID:
20006442.


2. Tian ZC, Liu GY, Shen H, Xie JR, Luo J, Tian MY. First report on the occurrence of
Rickettsia slovaca and
Rickettsia raoultii in
Dermacentor silvarum in China. Parasit Vectors 2012;5:19. PMID:
22257726.



3. Liu JZ, Liu ZN, Zhang Y, Yang XL, Gao ZH. Biology of
Dermacentor silvarum (Acari: Ixodidae) under laboratory conditions. Exp Appl Acarol 2005;36:131-138. PMID:
16082930.


4. de la Fuente J, Almazán C, Blas-Machado U, Naranjo V, Mangold AJ, Blouin EF, Gortazar C, Kocan KM. The tick protective antigen, 4D8, is a conserved protein involved in modulation of tick blood ingestion and reproduction. Vaccine 2006;24:4082-4095. PMID:
16580098.


5. de la Fuente J, Moreno-Cid JA, Canales M, Villar M, de la Lastra JM, Kocan KM, Galindo RC, Almazán C, Blouin EF. Targeting arthropod subolesin/akirin for the development of a universal vaccine for control of vector infestations and pathogen transmission. Vet Parasitol 2011;181:17-22. PMID:
21561715.


6. Galindo RC, Doncel-Pérez E, Zivkovic Z, Naranjo V, Gortazar C, Mangold AJ, Martín-Hernando MP, Kocan KM, de la Fuente J. Tick subolesin is an ortholog of the akirins described in insects and vertebrates. Dev Comp Immunol 2009;33:612-617. PMID:
19041667.


7. Moreno-Cid JA, Jiménez M, Cornelie S, Molina R, Alarcon P, Lacroix MN, Pinal R, Delacour S, Lucientes J, Canales M, de la Lastra JM, Villar M, de la Fuente J. Characterization of
Aedes albopictus akirin for the control of mosquito and sand fly infestations. Vaccine 2010;29:77-82. PMID:
20969924.


8. Carreón D, de la Lastra JM, Almazán C, Canales M, Ruiz-Fons F, Boadella M, Moreno-Cid JA, Villar M, Gortázar C, Reglero M, Villarreal R, de la Fuente J. Vaccination with BM86, subolesin and akirin protective antigens for the control of tick infestations in white tailed deer and red deer. Vaccine 2012;30:273-279. PMID:
22079077.


9. Merino O, Almazán C, Canales M, Villar M, Moreno-Cid JA, Galindo RC, de la Fuente J. Targeting the tick protective antigen subolesin reduces vector infestations and pathogen infection by
Anaplasma marginale and
Babesia bigemina. Vaccine 2011;29:8575-8579. PMID:
21951878.


10. Manzano-Román R, Díaz-Martín V, Oleaga A, Siles-Lucas M, Pérez-Sánchez R. Subolesin/akirin orthologs from
Ornithodoros spp. soft ticks: cloning, RNAi gene silencing and protective effect of the recombinant proteins. Vet Parasitol 2012;185:248-259. PMID:
22105082.


11. Bensaci M, Bhattacharya D, Clark R, Hu LT. Oral vaccination with vaccinia virus expressing the tick antigen subolesin inhibits tick feeding and transmission of
Borrelia burgdorferi. Vaccine 2012;30:6040-6046. PMID:
22864146.



12. Prudencio CR, de la Lastra JM, Canales M, Villar M, de la Fuente J. Mapping protective epitopes in the tick and mosquito subolesin ortholog proteins. Vaccine 2010;28:5398-5406. PMID:
20594626.


13. Moreno-Cid JA, de la Lastra JM, Villar M, Jiménez M, Pinal R, Estrada-Peña A, Molina R, Lucientes J, Gortázar C, de la Fuente J. Control of multiple arthropod vector infestations with subolesin/akirin vaccines. Vaccine 2013;31:1187-1196. PMID:
23291476.