The close association of wild animals and birds and their associated arthropod vectors with human populations and domestic livestock, travel, international trading of livestock, and illegal wildlife trading assist in the spread of infectious diseases worldwide [
1]. Deer serve as reservoirs of several vector-borne pathogens that impact on human and veterinary health throughout much of the world, including the Republic of Korea (ROK) [
2-
6]. In the ROK, the Korean water deer (KWD,
Hydropotes inermis argyropus) populations have rapidly increased from 1982 to 2011, in part, as a result of reforestation programs and the absence of predators and prohibition of hunting [
7]. Consequently, KWD are often observed grazing on vegetation near/in rural villages, agricultural areas (e.g., rice paddies), and even in metropolitan cities in close association with human populations, livestock, and poultry farms. A previous survey identified a high prevalence of
Anaplasma phagocytophilum,
Anaplasma bovis, and
Bartonella grahamii in KWD spleen tissues, suggesting that KWD are reservoirs of these pathogens [
8-
9].
A. phagocytophilum is the causative agent of human granulocytic anaplasmosis and transmitted mainly by
Ixodes ticks in many parts of the world [
10]. In the ROK,
A. phagocytophilum was identified by PCR in deer, rodents, and ticks belonging to the genera
Haemaphysalis and
Ixodes that were collected by tick drag and from migratory birds [
8,
11,
12].
B. grahamii was first isolated from rodents in the United Kingdom and is transmitted mainly by
Ctenocephalides nobilis nobilis, a flea often associated with rodents [
14,
15]. However, recently
Bartonella spp. have been detected in several tick species by PCR in other parts of the world [
16]. In the ROK,
Bartonella spp. have been detected in ticks, e.g.,
Haemaphysalis flava,
Haemaphysalis longicornis,
Ixodes nipponensis, and
Ixodes turdus, collected by tick drag and from migratory birds [
11,
13].
The purpose of this study was to identify tick-borne pathogens, e.g., A. phagocytophilum and Bartonella spp., in ticks that were collected from KWD that serve as suspected natural reservoirs of tickborne pathogens and pose serious vector-borne disease threats to wildlife, domestic animals, and human populations.
The Conservation Genome Resource Bank for Korean Wildlife examined KWD carcasses in the ROK from March 2008-May 2009. A total of 27/70 (38.6%) of the KWD carcasses were positive for ticks. Ticks were removed and placed individually in cryovials containing 70% ethyl alcohol and labeled with a unique identification number that corresponded to the KWD collection data. Ticks were provided to the 5th Medical Detachment, 168th Multifunctional Medical Battalion, 65th Medical Brigade, Yongsan US Army Garrison, Seoul, where they were identified [
17] and pooled according to tick species, life stages, sex (adults), and KWD collection numbers and then provided to the Laboratory of Veterinary Internal Medicine, Seoul National University where they were tested for selected pathogens.
Individuals and pools of ticks were homogenized mechanically using a Beadbeater TissueLyser II (QIAGEN, Hilden, Germany) with lysis buffer, proteinase K, and 5 mm stainless steel beads (30 frequencies/s for 5 min), followed by incubation at 56˚C overnight and then centrifugation at 12,000 g for 10 min at 4˚C. The genomic DNA extraction was performed using DNeasy
® Tissue Kits (QIAGEN) according to the manufacturer’s instructions. PCR and nested-PCR were performed using generic-specific primer sets for
Bartonella and
Ehrlichia/Anaplasma, and species-specific primers for
A. phagocytophilum,
A. bovis, and
B. grahamii as described by Kang et al. [
13].
PCR products were purified using QIAquick Gel Extraction kits (QIAGEN) and were cloned with pGEM
®-T Easy Vectors (Promega Corp.). Plasmid DNA for sequencing was purified using the Wizard
® Plus SV Minipreps DNA Purification System (Promega Corp.) according to the manufacturer’s instructions. Purified recombinant plasmid DNA was sequenced by dideoxy termination with an automatic sequencer (ABI 3730xl capillary DNA sequencer, Foster City, California, USA). The DNA sequences were evaluated with Chromas software (Ver 2.33), aligned using Clustal X (Ver 2.1), and then examined with a similarity matrix. The phylogenetic trees of nucleotide sequences were developed using the MEGA6 program. GenBank accession numbers for the 16S ribosomal (r) RNA and ITS region sequences and specific genospecies sequences related to bacterial pathogens for sequence comparisons are shown in
Figs. 1,
2.
A total of 363 (21 larvae, 114 nymphs, and 228 adults) fed ticks belonging to 2 genera and 4 species,
H. flava (n=87),
H. longicornis (n=228),
I. nipponensis (n=8), and
I. persulcatus (n=40), were collected from 27/70 (38.6%) KWD carcasses (
Table 1). The minimum infection rate of
A. phagocytophilum was 24.5% (89/363 ticks, 266 pools) for ticks using nested PCR targeting the 16S rRNA gene (
Table 1). The 16S rRNA sequences (n=89) of
A. phagocytophilum were classified into 5 genotypes that showed 98.7-100% similarity to the
A. phagocytophilum HZ strain isolated from a human (CP000235) (
Fig. 1). Four of 5 genotypes (KWDTAP1, 2, 3, and 4) corresponded to previously detected 16S rRNA sequences of
A. phagocytophilum (KWDAP1, 2, 3, and 4) from KWD spleen tissues [
8]. In addition, the 5/363 (1.4%) amino acid sequences of
A. phagocytophilum groEL (KWDTAPg, JQ086319) obtained from 2
H. flava, 2
I. nipponensis, and 1
I. persulcatus adult ticks were identical to each other and corresponded to the amino acid sequence of
A. phagocytophilum groEL (ADO34908) that was previously reported from KWD spleen tissue. All
A. bovis 16S rRNA gene sequences (JQ086318) from ticks were identical and corresponded to the
A. bovis sequence (GU556626) previously identified from KWD spleen tissues. A total of 13 (3.6%) of the ticks demonstrated dual infections with
A. phagocytophilum and
A. bovis.
Using the ITS specific nested PCR, a total of 11
Bartonella sequences were classified into 2 genotypes (KWD-Ba1 and KWDBa2) (
Fig. 2). KWDBa1 (JQ086210, n=4) was identified as
B. grahamii V2 strain (AJ269785) and corresponded to the previous ITS sequence (JN810845) identified from KWD spleen tissues. KWDBa2 (JQ086321, n=7), which showed 92.7% similarity to
B. grahamii V2 strain (AJ269785), was identified as
Bartonella spp. (
Fig. 2). In addition, 2 (0.6%) adult ticks each demonstrated double infections with
A. phagocytophilum and
Bartonella spp., while 1 (0.3%) individual adult tick demonstrated triple infections with
A. phagocytophilum,
A. bovis, and
Bartonella spp.
KWD and the Korean spotted deer (
Cervus nippon mantchuricus) are strongly suspected as reservoirs of
Anaplasma and
Bartonella species in the ROK [
6,
11,
13]. This study supports evidence that ticks become infected while blood feeding on deer and are vectors of
Anaplasma and
Bartonella species that are present in deer populations in the ROK. However, there is insufficient correlation of
Anaplasma and
Bartonella spp. transmission between KWD and blood feeding ticks since some attached ticks collected from
A. phagocytophilum positive KWD were negative for
A. phagocytophilum, while attached ticks collected from negative KWD were positive for
A. phagocytophilum. These results indicate that blood feeding does not always accompany ingestion of these vector-borne pathogens.
Bartonella spp. are mainly transmitted by fleas, but recently
Bartonella DNA has been detected in unfed
Haemaphysalis and
Ixodes spp. collected by tick drag and from partially fed and recently attached ticks collected from migratory birds in the ROK [
11,
13]. Molecular evidence supports the potential transmission of
Bartonella spp. since it is transmitted by transstadial transmission as indicated by positive unfed ticks that were collected by tick drag. Tick bites among ROK populations are largely unreported with an average of only 40 tick bites annually, while there were 55 cases of severe fever with thrombocytopenia syndrome reported for 2014 [
18]. Therefore, the potential risk associated with the transmission of
A. phagocytophilum and
Bartonella spp. in ticks that fed on infected KWD to ROK civilian and military populations and livestock is unknown.
In summary, evidence supports the maintenance of A. phagocytophilum, Bartonella spp. and other tick-borne pathogens in KWD and associated ticks that pose potential serious threats to medical and veterinary health in the ROK.
ACKNOWLEDGEMENTS
This work was carried out with the support of “Cooperative Research Program for Agriculture Science & Technology Development (project no. PJ010092)”, Rural Development Administration, the Republic of Korea. This research was also supported by a National Research Foundation of Korea Grant funded by the Korean Government (2011-0015349), the Public Health Command District-Korea, and the Armed Forces Health Surveillance Branch, Global Emerging Infections Surveillance and Response System (AFHSC-GEIS), Silver Spring, Maryland, USA.
Disclosure: The opinions expressed in this article are those of the authors and do not reflect official policy or positions of the US Department of the Army, the US Department of Defense, or the US Government.
CONFLICTS OF INTEREST
We have no conflict of interest related to this work.
REFERENCES
1. Bengis RG, Leighton FA, Fischer JR, Artois M, Mörner T, Tate CM. The role of wildlife in emerging and re-emerging zoonoses. Rev Sci Tech 2004;23:497-511.

2. Belongia EA, Reed KD, Mitchell PD, Kolbert CP, Persing DH, Gill JS, Kazmierczak JJ. Prevalence of granulocytic
Ehrlichia infection among white-tailed deer in Wisconsin. J Clin Microbiol 1997;35:1465-1468.


3. Little SE, Stallknecht DE, Lockhart JM, Dawson JE, Davidson WR. Natural coinfection of a white tailed deer (
Odocoileus virginianus) population with three
Ehrlichia spp. J Parasitol 1998;84:897-901.


4. Petrovec M, Bidovec A, Sumner JW, Nicholson WL, Childs JE, Avsic-Zupanc T. Infection with Anaplasma phagocytophila in cervids from Slovenia: evidence of two genotypic lineages. Wien Klin Wochenschr 2002;31:641-647.
5. Kawahara M, Rikihisa Y, Lin Q, Isogai E, Tahara K, Itagaki A, Hiramitsu Y, Tajima T. Novel genetic variants of
Anaplasma phagocytophilum,
Anaplasma bovis,
Anaplasma entral, and a novel
Ehrlichia sp. in wild deer and ticks on two major islands in Japan. Appl Environ Microbiol 2006;72:1102-1109.



6. Lee M, Yu D, Yoon J, Li Y, Lee J, Park J. Natural co-infection of
Ehrlichia chaffeensis and
Anaplasma bovis in a deer in South Korea. J Vet Med Sci 2009;71:101-103.


7. National Institute of Biological Resources of Korea (NIBR). Survey and resource management of wildlife. National Institute of Biological Resources of Korea. Incheon, Korea. 2011, p 24.
8. Kang JG, Ko S, Kim YJ, Yang HJ, Lee H, Shin NS, Choi KS, Chae JS. New genetic variants of
Anaplasma phagocytophilum and
Anaplasma bovis from Korean water deer (
Hydropotes inermis argyropus). Vector-Borne Zoonotic Dis 2011;11:929-938.


9. Ko S, Kim SJ, Kang JG, Won S, Lee H, Shin NS, Choi KS, Youn HY, Chae JS. Molecular detection of
Bartonella grahamii and
B. schoenbuchensis-related species in Korean water deer (
Hydropotes inermis argyropus). Vector-Borne Zoonotic Dis 2013;13:415-418.


10. Chen SM, Dumler JS, Bakken JS, Walker DH. Identification of a granulocytotropic
Ehrlichia species as the etiologic agent of human disease. J Clin Microbiol 1994;32:589-595.


11. Kim CM, Kim JY, Yi YH, Lee MJ, Cho MR, Shah DH, Klein TA, Kim HC, Song JW, Chong ST, O’Guinn ML, Lee JS, Lee IY, Park JH, Chae JS. Detection of
Bartonella species from ticks, mites and small mammals in Korea. J Vet Sci 2005;6:327-334.

12. Oh JY, Moon BC, Bae BK, Shin EH, Ko YH, Kim YJ, Park YH, Chae JS. Genetic identification and phylogenetic analysis of
Anaplasma and
Ehrlichia species in
Haemaphysalis longicornis collected from Jeju Island, Korea. J Bacteriol Virol 2009;39:1-11.

13. Kang JG, Kim HC, Choi CY, Nam HY, Chae HY, Chong ST, Klein TA, Ko S, Chae JS. Molecular detection of
Anaplasma,
Bartonella, and
Borrelia species in ticks collected from migratory birds from Hong-do Island, Republic of Korea. Vector-Borne Zoonotic Dis 2013;13:215-225.


14. Birtles RJ, Harrison TG, Saunders NA, Molyneux DH. Proposals to unify the genera
Grahamella and
Bartonella, with descriptions of
Bartonella talpae comb. nov.,
Bartonella peromysci comb. nov., and three new species,
Bartonella grahamii sp. nov.,
Bartonella taylorii sp. nov., and
Bartonella doshiae sp. nov. Int J Syst Bacteriol 1995;45:1-8.


15. Bown JK, Bennett M, Begon M. Flea-borne
Bartonella grahamii and
Bartonella taylorii in bank voles. Emerg Infect Dis 2004;10:684-687.



16. Billeter SA, Levy MG, Chomel BB, Breitschwerdt EB. Vector transmission of
Bartonella species with emphasis on the potential for tick transmission. Med Vet Entomol 2008;22:1-15.


17. Yamaguti N, Tipton VJ, Keegan HL, Toshioka S. Ticks of Japan, Korea, and the Ryukyu Islands. Brigham Young University Science Bulletin, Biological Series 1971;15:1-226.

18. Korea Centers for Disease Control and Prevention. Infectious Diseases Surveillance Yearbook. 2014, p 27.
Fig. 1.
Phylogenetic relationships for Anaplasma phagocytophilum (bold letters) detected from ticks collected from Korean water deer and Anaplasma and Ehrlichia species based on partial nucleotide sequences of 16S rRNA gene fragments (926 bp). The neighbor-joining method was used for constructing the phylogenetic tree. The numbers at the nodes are the proportions of 1,000 bootstrap iterations that support the topology shown.
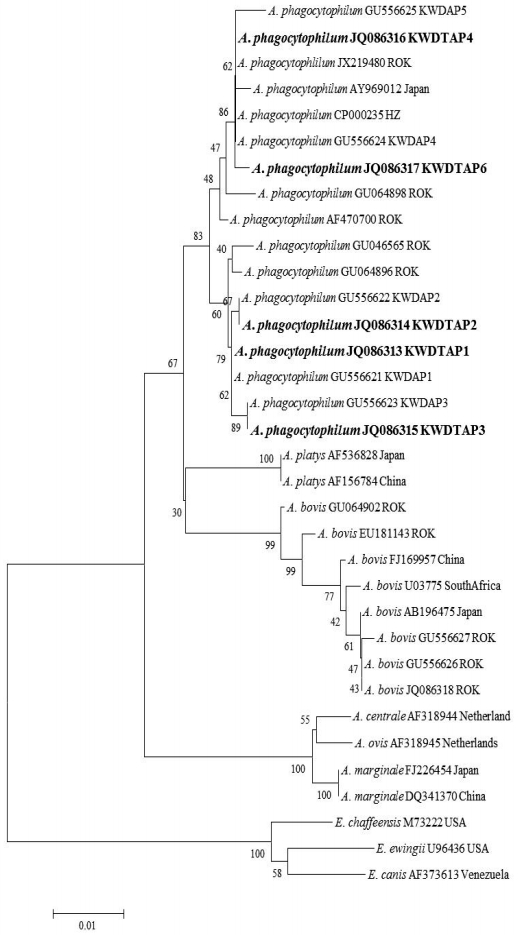
Fig. 2.
Phylogenetic relationships for Bartonella grahamii and Bartonella spp. (bold letters) detected from ticks collected from Korean water deer and Bartonella spp. based on partial nucleotide sequences of ITS gene fragments (484 bp). The neighbor-joining method was used for constructing the phylogenetic tree. The numbers at the nodes are the proportions of 1,000 bootstrap iterations that support the topology shown.
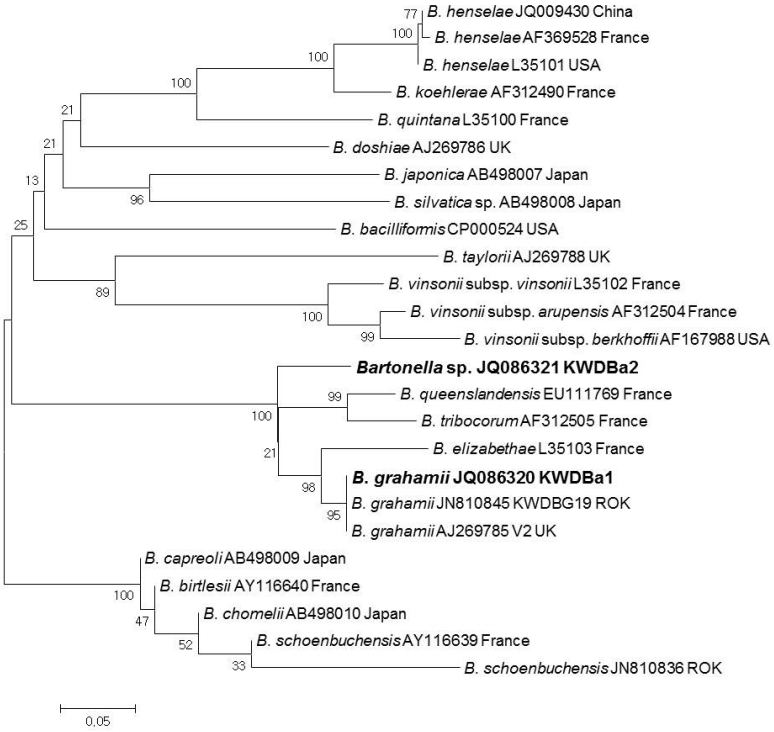
Table 1.
Prevalence of tick-borne pathogens in fed ticks collected from Korean water deer (Hydropotes inermis argyropus) in the ROK using 16S rRNA primer sets
|
Species |
Stage |
No. of pools (no. of ticks) |
No. of PCR-positive samples (prevalence [%]a)
|
|
A. phagocytophilum
|
A. bovis
|
Bartonella spp. |
Double infectionb
|
Double infectionc
|
Triple infectiond
|
|
H. flava
|
Larva |
1 (1) |
0 |
0 |
0 |
0 |
0 |
0 |
|
Nymph |
8 (10) |
3 (30.0) |
0 |
2 (20.0) |
0 |
0 |
0 |
|
Male |
47 (47) |
14 (29.8) |
3 (6.4) |
1 (2.1) |
3 (6.4) |
0 |
0 |
|
Female |
29 (29) |
13 (44.8) |
2 (6.9) |
2 (6.9) |
1 (3.4) |
0 |
0 |
|
Subtotal |
85 (87) |
30 (34.5) |
5 (5.8) |
5 (5.8) |
4 (4.6) |
0 |
0 |
|
H. longicornis
|
Larva |
2 (19) |
2 (10.5) |
0 |
0 |
0 |
0 |
0 |
|
Nymph |
26 (104) |
8 (7.7) |
1 (1.0) |
2 (1.9) |
1 (1.0) |
1 (1.0) |
0 |
|
Male |
45 (45) |
7 (15.6) |
2 (4.5) |
0 |
1 (2.2) |
0 |
0 |
|
Female |
60 (60) |
21 (35.0) |
9 (15.0) |
3 (5.0) |
5 (8.3) |
1 (1.7) |
0 |
|
Subtotal |
133 (228) |
38 (16.7) |
12 (5.3) |
5 (2.2) |
7 (3.0) |
2 (0.9) |
0 |
|
I. nipponensis
|
Male |
5 (5) |
1 (20.0) |
0 |
0 |
0 |
0 |
0 |
|
Female |
3 (3) |
2 (66.7) |
1 (33.3) |
0 |
1 (33.3) |
0 |
0 |
|
Subtotal |
8 (8) |
3 (37.5) |
1 (12.5) |
0 |
1 (12.5) |
0 |
0 |
|
I. persulcatus
|
Larva |
1 (1) |
0 |
0 |
0 |
0 |
0 |
0 |
|
Male |
15 (15) |
8 (53.3) |
0 |
0 |
0 |
0 |
0 |
|
Female |
24 (24) |
10 (41.7) |
2 (8.3) |
1 (4.2) |
1 (4.2) |
0 |
1 (4.2) |
|
Subtotal |
40 (40) |
18 (45.0) |
2 (5.0) |
1 (2.5) |
1 (2.5) |
0 |
1 (2.5) |
|
Total |
Larva |
4 (21) |
2 (9.5) |
0 |
0 |
0 |
0 |
0 |
|
Nymph |
34 (114) |
11 (9.6) |
1 (0.9) |
4 (3.5) |
0 |
0 |
0 |
|
Male |
112 (112) |
30 (26.8) |
5 (4.5) |
1 (0.9) |
4 (3.6) |
0 |
0 |
|
Female |
116 (116) |
46 (39.7) |
14 (12.1) |
6 (5.2) |
9 (7.8) |
0 |
1 (0.9) |
|
Total |
266 (363) |
89 (24.5) |
20 (5.5) |
11 (3.0) |
13 (3.6) |
2 (0.6) |
1 (0.3) |



