Cited By
Citations to this article as recorded by

Molecular detection of the zoonotic trematode Centrocestus formosanus (Nishigori, 1924) (Opisthorchiida, Heterophyidae) in Central Europe
Ľubomír Šmiga, Júlia Šmigová, Federica Berrilli, Ingrid Papajová, Peter Lazár, Isabel Guadano-Procesi
Veterinary Research Communications.2025;[Epub]
CrossRef Foodborne intestinal flukes: A brief review of epidemiology and geographical distribution
Jong-Yil Chai, Bong-Kwang Jung
Acta Tropica.2020; 201: 105210.
CrossRef Multiplex PCR development for the simultaneous and rapid detection of two pathogenic flukes, Dactylogyrus spp. and Centrocestus formosanus, in ornamental fishes
Phonkawin Jaruboonyakorn, Thanawan Tejangkura, Thapana Chontananarth
General overview of the current status of human foodborne trematodiasis
Jong-Yil Chai, Bong-Kwang Jung
Parasitology.2022; 149(10): 1262.
CrossRef Evidence of Centrocestus formosanus (Nishigori, 1924) in Zebrafish (Danio rerio)
Carmelo Iaria, Sergio Migliore, Daniele Macri, Maurizio Bivona, Fabiano Capparucci, Gabriella Gaglio, Fabio Marino
First Record ofClinostomumsp. (Digenea: Clinostomidae) inDanio rerio(Actinopterygii: Cyprinidae) and the Implication of Using Zebrafish from Pet Stores on Research
Tony Silveira, Mateus T. Kütter, Camila M.G. Martins, Luis Fernando Marins, Robert T. Boyle, Vinicius F. Campos, Mariana H. Remião
Loop‐Mediated Isothermal Amplification Combined With Lateral‐Flow Dipstick for Detection of Centrocestus formosanus in Ornamental Fish
Metawee Sabaijai, Thanawan Tejangkura, Thapana Chontananarth
Journal of Fish Diseases.2025;[Epub]
CrossRef Delineating the origins of the multidrug-resistant pathogens in ornamental fish farms by multilocus sequence typing and identification of a novel multidrug-resistant plasmid
Songzhe Fu, Ping Ni, Yi Wang, Shibo Jin, Zhiqiang Jiang, Shigen Ye, Ruijun Li
Canadian Journal of Microbiology.2019; 65(8): 551.
CrossRef The life cycle of a zoonotic parasite reassessed: Experimental infection of Melanoides tuberculata (Mollusca: Thiaridae) with Centrocestus formosanus (Trematoda: Heterophyidae)
Hudson A. Pinto, Nicole Q. Gonçalves, Danimar López-Hernandez, Eduardo A. Pulido-Murillo, Alan L. Melo, Petr Heneberg
PLOS ONE.2018; 13(4): e0194161.
CrossRef Good practices in the rearing and maintenance of zebrafish (Danio rerio) in Brazilian laboratories
Mateus Tavares Kütter, Leonardo José Gil Barcellos, Robert Tew Boyle, Luis Fernando Marins, Tony Silveira
Ciência Animal Brasileira.2023;[Epub]
CrossRef Boas práticas na criação e manutenção de zebrafish (Danio rerio) em laboratório no Brasil
Mateus Tavares Kütter, Leonardo José Gil Barcellos, Robert Tew Boyle, Luis Fernando Marins, Tony Silveira
Ciência Animal Brasileira.2023;[Epub]
CrossRef Infections of Digenetic Trematode Metacercariae in Wrestling Halfbeak, Dermogenys pusilla from Bangkok Metropolitan Region in Thailand
Laddawan Patarwut, Thapana Chontananarth, Jong-Yil Chai, Watchariya Purivirojkul
The Korean Journal of Parasitology.2020; 58(1): 27.
CrossRef Diagnosis of Centrocestus formosanus Infection in Zebrafish (Danio rerio) in Italy: A Window to a New Globalization-Derived Invasive Microorganism
Antonino Pace, Ludovico Dipineto, Serena Aceto, Maria Concetta Censullo, Maria Carmen Valoroso, Lorena Varriale, Laura Rinaldi, Lucia Francesca Menna, Alessandro Fioretti, Luca Borrelli
Abstract
The prevalence of Centrocestus formosanus metacercariae was investigated in ornamental fish purchased from a pet shop in Chiang Mai, Thailand, including Carassius auratus (goldfish), Cyprinus carpio (Koi), Poecilia latipinna (Sailfin Molly), Danio rerio (Zebrafish), and Puntigrus tetrazona (Tiger barb). The parasite species was identified by the morphology of worms as well as by a molecular approach using ITS2. The results showed that 50 (33.3%) of 150 fish examined were infected with the metacercariae. The highest prevalence was found in C. auratus (83.3%), and the highest intensity was noted in C. carpio (70.8 metacercariae/fish). The most important morphological character was the presence of 32–34 circumoral spines on the oral sucker. The phylogenetic studies using the rRNA ITS2 region revealed that all the specimens of C. formosanus in this study were grouped together with C. formosanus in GenBank database. This is the first report on ornamental fish, C. carpio, P. latipinna, D. rerio, and P. tetrazona, taking the role of second intermediate hosts of C. formosanus in Thailand. Prevention and control of metacercarial infection in ornamental fish is urgently needed.
Key words: Centrocestus formosanus, ornamental fish, Carassius auratus, ITS2, Chiang Mai
Centrocestus formosanus (Nishigori, 1924) (Heterophyidae) was described from Taiwan and now known to distribute widely in Asia [
1]. The adult worm lives in the intestine of fish-eating birds and mammals [
2–
4]. Human cases infected with
C. formosanus were reported in Lao PDR and Vietnam [
4,
5]. Pleurolophocercous cercariae are shed from the thiarid snail (
Melanoides tuberculata) [
1,
6]. The metacercariae are found on the gill of several freshwater fish species, such as
Puntius brevis, Hampala dispar, Puntius gonionotus, Puntius meiacanthus, Cyclocheilichthys armatus, Anabas testudineus, and
Henicorhynchus siamensis [
7–
9]. In addition, the ornamental fish were reported to be infected with
C. formosanus [
2]. The parasite infection causes a problem in fish culture and leads to a reduction of fish production in aquaculture [
1,
10]. The presence of metacercariae on the gills of fish could be one of the reasons for the death of fish [
2]. In Thailand, 2 species of
Centrocestus, including
C. formosanus and
C. caninus, were reported; however,
C. caninus is regarded as a synonym of
C. formosanus [
4].
The adult stage of this fluke was found in humans from Chiang Mai and Chiang Rai Provinces, northern Thailand [
11,
12]. The metacercariae were found in several species of freshwater fish [
7,
13–
17]. However, there were few studies on metacercarial infection in ornamental fish species. Thus, the objective of this study was to determine the prevalence and species identification of
Centrocestus in ornamental fish purchased from a pet shop in Chiang Mai Province, northern Thailand through morphological and molecular studies.
Total 150 ornamental fish, including 30
Carassius auratus, 30
Cyprinus carpio, 30
Poecilia latipinna, 30
Danio rerio, and 30
Puntigrus tetrazona, were collected from a pet shop in the Mueang District, Chiang Mai Province, northern Thailand during May–June 2016. The metacercariae were investigated on the gill of fish under a stereomicroscope, and then all the mature metacercariae (with X-shape excretory bladder) were collected for further studies. Some of the metacercariae were fed to chicks (1-day old), and after 7 days the adult stages were collected from their small intestines. Some other metacercariae were used for molecular studies. The adult stage was used to make a permanent slide according to Boonchot et al. [
18]. All specimens were fixed with 4% formalin, stained with hematoxylin, dehydrated in alcohol series, and finally mounted in permount.
C. formosanus genomes were extracted by Chelex (Fluka, Sigma-Aldrich, St. Louis, Missouri, USA) following Caron et al. [
19]. DNA products were amplified with ITS2 region. The reactions were performed in a Thermal Cycler machine (Little Genius, Bioer Technology, Tokyo, Japan). The primer combination; forward 3S (5′-GGT ACC GGT GGA TCA CTC GGC TCG TG-3′) and reverse BD2 (5′-TAT GCT TAA ATT CAG CGG GT-3′) were performed in PCR for ITS2 gene. The PCR conditions were as follows: 2 min initial denaturation at 94°C, followed by 35 cycles of 1 min DNA denaturation at 94°C, 1 min primer annealing at 60°C, 1 min extension at 72°C, and 7 min for final extension at 72°C. PCR products were tested by gel electrophoresis with DNA Dye NonTox (Applichem, Darmstadt, Germany) stain. After gel electrophoresis, PCR products were purified and sent to sequence analysis. The sequence was analyzed by ClustalW in MEGA software version 6.0 [
20]. The sequence of
C. formosanus was compared and checked by BLAST program on National Center for Biotechnology Information database for species confirmation and gathering of essential sequences for phylogenetic analysis. ITS2 sequences of
C. formosanus,
Centrocestus sp.,
Haplorchis taichui, Haplorcoides sp.,
Stellantchasmus falcatus, Paramphistomum epiclitum, Fasciola gigantica, and
Heterakis gallinarum from GenBank were aligned with
C. formosanus from this study. The phylogenetic tree was constructed using the MEGA software version 6.0. The data was analyzed by character analysis (maximum-likelihood) and distance analysis (neighbor-joining) with 1,000 bootstrap values.
Total 50 (33.3%) of 150 ornamental fish examined were infected with metacercariae of
C. formosanus. The highest prevalence was found in
C. auratus (83.3%), and the highest intensity of infection was in
C. carpio (av. 70.8 metacercariae per fish). The results of other fish are shown in
Table 1. The measurements of adult worms are shown in
Table 2. The morphology of
C. formosanus from 5 ornamental fish was almost similar. The metacercariae were oval shaped, with X-shaped excretory bladder and 32–34 circumoral spines surrounding the oral sucker. The adult flukes were pyriform shaped, and the tegument was covered with scale like spines. All adult worms originating from
C. auratus possessed exclusively 34 circumoral spines (
Fig. 1A1, A2), whereas those worms originating from other fish were armed with 32 spines arranged in 2 rows (
Fig. 1B1, B2). They were both regarded as
C. formosanus.
The BLAST results of
C. formosanus originating from
C. auratus of this study showed 99% similarity with 5.8S rRNA gene (partial sequence), ITS2 of
C. formosanus GenBank accession no. KJ630863. The phylogenetic trees were reconstructed based on a maximum-likelihood and neighbor-joining methods with bootstrap values of 1,000 replicates. The results from both methods showed that the topology is similar (
Fig. 2) to that of
C. formosanus originating from
C. auratus [
25] which was grouped with
Centrocestus sp. and
C. formosanus from GenBank with 100% and 98% bootstrap values, respectively.
C. formosanus metacercariae were found in ornamental fish from many countries, such as Mexico, Australia, Denmark, and Iran [
21–
24]. In Thailand, the metacercariae of
C. formosanus have been reported in several fish species, such as
Macrognathus siamensis, P. gonionotus, P. brevis, Thynnichthys thynnoides, Puntioplites proctozysron, Esomus metallicus, A. testudineus, Parambassis siamensis, and
Hampala macrolepidota [
14,
25,
26]. However, this fluke is not well known in ornamental fish in Thailand, and in Chiang Mai Province, it has been reported only in
C. auratus [
25,
27]. In this study, the metacercariae of
C. formosanus were found in 5 species of ornamental fish. The results elucidated that ornamental fish can serve as the second intermediate host for
C. formosanus. In addition,
C. formosanus metacercariae were found for the first time in
C. carpio, D. rerio, P. latipinna and
P. tetrazona in Thailand.
The species confirmation of
C. formosanus was based on morphological and molecular methods. The unique character of
C. formosanus is the number of circumoral spines around the oral sucker. They commonly have 32 circumoral spines arranged in 2 rows around the oral sucker [
1,
4,
8,
18,
24,
28], whereas
Centrocestus armatus has 42–44 circumoral spines [
3,
29,
30]. In the present study, there were 32 circumoral spines in worms originating from 4 fish species (
C. carpio, P. latipinna, D. rerio, and
P. tetrazona) which agreed to previous studies [
1,
2,
24,
28]. The worms originating from
C. auratus fish had exclusively 34 circumoral spines which also resembled previous studies [
8,
21]. Thus, our specimens of 2 different origins were both considered morphologically to be
C. formosanus. Molecular studies of our specimens using ITS2 by maximum-likelihood and neighbor-joining methods revealed a high relationship with
C. formosanus from GenBank. They were separated from other heterophyid flukes and also from
P. epiclitum, F. gigantica, and
H. gallinarum (out group). The results of morphological and molecular studies were accorded.
This is the first report of C. formosanus metacercariae in C. carpio, P. latipinna, D. rerio, and P. tetrazona ornamental fish. The phylogenetic trees showed high relationships of our specimens with C. formosanus from GenBank database. Infection of these fish with C. formosanus metacercariae should be prevented and controlled. It can also help to reduce the motility rate of fish before export or sell to customers.
ACKNOWLEDGMENTS
We would like to thank Environmental Science Research Center (ESRC), Chiang Mai University, Chiang Mai, Thailand. Special thanks to the staff of the Applied Parasitology Research Laboratory, Department of Biology, Faculty of Science, and Chiang Mai University for the facility supported. We are also thankful to the Science and Technology Research Institute, Chiang Mai University for financial support.
CONFLICTS OF INTEREST
CONFLICT OF INTEREST
We have no conflict of interest related to this study.
REFERENCES
1. Scholz T, Salgado-Maldonado G. The introduction and dispersal of
Centrocestus formosanus (Nishigori, 1924) (Digenea: Heterophyidae) in Mexico: a review.
Am Midl Nat 2000;143:185-200.

2. Gjurčević E, Petrinec Z, Kozariž Z, Kužir S, Gjurčević KV, Vučemilo M, Džaja P. Metacercariae of Centrocestus formosanus in goldfish (Carassius auratus L.) imported into Croatia. Helminthologia 2007;4:214-216.
3. Kimura D, Paller VG, Uga S. Development of
Centrocestus armatus in different final hosts.
Vet Parasitol 2007;146:367-371.


4. Chai JY, Sohn WM, Yong TS, Eom KS, Min DY, Lee MY, Lim H, Insisiengmay B, Phommasack B, Rim HJ.
Centrocestus formosanus (Heterophyidae): human infections and the infection source in Lao PDR.
J Parasitol 2013;99:531-536.


5. De NV, Le TH. Human infections of fish-borne trematodes in Vietnam: prevalence and molecular specific identification at an endemic commune in Nam Dinh Province.
Exp Parasitol 2011;129:355-361.


6. Pinto HA, de Melo AL.
Melanoides tuberculata (Mollusca: Thiaridae) as an intermediate host of
Centrocestus formosanus (Trematoda: Heterophyidae) in Brazil.
Rev Inst Med Trop São Paulo 2010;52:207-210.


7. Srisawangwong T, Sithithaworn P, Tesana S. Metacercariae isolated from cyprinoid fish in Khon Kaen district by digestion technique.
Southeast Asian J Trop Med Public Health 1997;28:224-226.

8. Han ET, Shin EH, Phommakorn S, Sengvilaykham B, Kim JL, Rim HJ, Chai JY.
Centrocestus formosanus (Digenea: Heterophyidae) encysted in the freshwater fish,
Puntius brevis, from Lao PDR.
Korean J Parasitol 2008;46:49-53.



9. Eom KS, Park HS, Lee D, Sohn WM, Yong TS, Chai JY, Min DY, Rim HJ, Insisiengmay B, Phommasack B. Infection status of zoonotic trematode metacercariae in fishes from Vientiane Municipality and Champasak Province in Lao PDR.
Korean J Parasitol 2015;53:447-453.



10. Paller VGV, Uga S. Attachment and penetration of
Centrocestus armatus (Digenea: Heterophyidae) cercariae to gills of secondary intermediate fish hosts.
J Parasitol 2008;93:578-583.

11. Radomyos B, Wongsaroj T, Wilairatana P, Radomyos P, Praevanich R, Meesomboon V, Jongsuksuntikul P. Opisthorchiasis and intestinal fluke infections in northern Thailand.
Southeast Asian J Trop Med Public Health 1998;29:123-127.


12. Waikagul J, Wongsaroj T, Radomyos P, Meesomboon V, Praewanich R, Jongsuksuntikul P. Human infection of
Centrocestus caninus in Thailand.
Southeast Asian J Trop Med Public Health 1997;28:831-835.

13. Waikagul J.
Opisthorchis viverrini metacercaria in Thai freshwater fish.
Southeast Asian J Trop Med Public Health 1998;29:324-326.

14. Wongsawad C, Rojtinnakorn J, Wongsawad P, Rojanapaibul A, Marayong T, Suwattanacoupt S, Sirikanchana P, Sey O, Jadhav BV. Helminths of vertebrates in Mae Sa Stream, Chiang Mai, Thailand.
Southeast Asian J Trop Med Public Health 2004;35:140-146.

15. Saenphet S, Wongsawad C, Saenphet K, Rojanapaibul A, Vanittanakom P, Chai JY. The occurrence of heterophyid metacercariae in cyprinoid fish in Chiang Mai province. Southeast Asian J Trop Med Public Health 2008;39:56-61.
16. Wongsawad C, Wongsawad P, Anuntalabhochai S, Chai JY, Sukontason K. Occurrence and molecular identification of liver and minute intestinal flukes metacercariae in freshwater fish from Fang-Mae Ai Agricultural Basin, Chiang Mai province, Thailand. Asian Biomed 2013;7:97-104.
17. Krailas D, Veeravechsukij N, Chuanprasit C, Boonmekam D, Namchote S. Prevalence of fish-borne trematodes of the family Heterophyidae at Pasak Cholasid Reservoir, Thailand.
Acta Trop 2016;156:79-86.


18. Boonchot K, Wongsawad C. A survey of helminthes in cyprinoid fish from the Mae Ngad Somboonchon reservoir, Chiang Mai Province, Thailand.
Southeast Asian J Trop Med Public Health 2005;36:103-107.

19. Caron Y, Righib S, Lempereura L, Saegermanc C, Lossona B. An optimized DNA extraction and multiplex PCR for the detection of
Fasciola sp. in lymnaeid snails.
Vet Parasitol 2011;178:93-99.


20. Tamaru K, Stecher G, Peterson D, Filipski A, Kumar S. Mega6: molecular evolutionary genetics analysis version 6.0.
Mol Biol Evol 2013;30:2725-2729.



21. Ortega C, Fajardo R, Enríquez R. Trematode
Centrocestus formosanus infection and distribution in ornamental fishes in Mexico.
J Aquat Anim Health 2009;21:18-22.


22. Evans BB, Lester RJG. Parasites of ornamental fish imported into Australia. Bull Eur Ass Fish Pathol 2001;21:51-55.
23. Mehrdana F, Jensen HM, Kania PW, Buchmann K. Import of exotic and zoonotic trematodes (Heterophyidae:
Centrocestus sp.) in
Xiphophorus malculatus: implications for ornamental fish import control in Europe.
Acta Parasitol 2014;59:276-283.

24. Mood SM, Ebrahimzadeh Mousavi HA, Mokhayer B, Ahmadi M, Soltani M, Sharifpour I. Centrocestus formosanus metacercarial infection of four ornamental fish species imported into Iran. Bull Eur Ass Fish Pathol 2010;30:146-149.
25. Wongsawad C, Wongsawad P, Sukontason K, Maneepitaksanti W, Nantarat N. Molecular phylogenetics of
Centrocestus formosanus (Digenea: Heterophyidae) originated from freshwater fish from Chiang Mai Province, Thailand.
Korean J Parasitol 2017;55:31-37.



26. Sukontason K, Piangjai S, muangyimpong Y, Sukontason K, Methanitikorn R, Chaithong U. Prevalence of trematode metacercariae in cyprinoid fish of Ban Pao District, Chiang Mai province, northern Thailand.
Southeast Asian J Trop Med Public Health 1999;30:365-370.

27. Saenphet S, Wongsawad C, Saenphet K, Chai JY. Mucosal mast cell responses in rats (
Rattus norvegicus) experimentally infected with
Centrocestus caninus.
Southeast Asian J Trop Med Public Health 2006;37:446-451.

28. Mati LVT, Pinto HA, Melo Al. Experimental infection of Swiss and AKR/J mice with
Centrocestus formosanus (Trematoda: Heterophyidae).
Rev Inst Med Trop Sao Paulo 2013;55:13-36.


29. Hong SJ, Seo BS, Lee SH, Chai JY. A human case of
Centrocestus armatus infection in Korea.
Korean J Parasitol 1988;26:55-60.

30. Kimura D, Uga S. Epidemiological study on
Centrocestus armatus metacercariae in the Chikusa River, Hyogo Prefecture, Japan.
Trop Med Health 2005;33:7-11.

Fig. 1
Line drawing of an adult Centrocestus formosanus originating from C. auratus (A1) showing 34 circumoral spines around the oral sucker (A2) and another originating from C. carpio (B1) showing 34 circumoral spines around the oral sucker (B2).
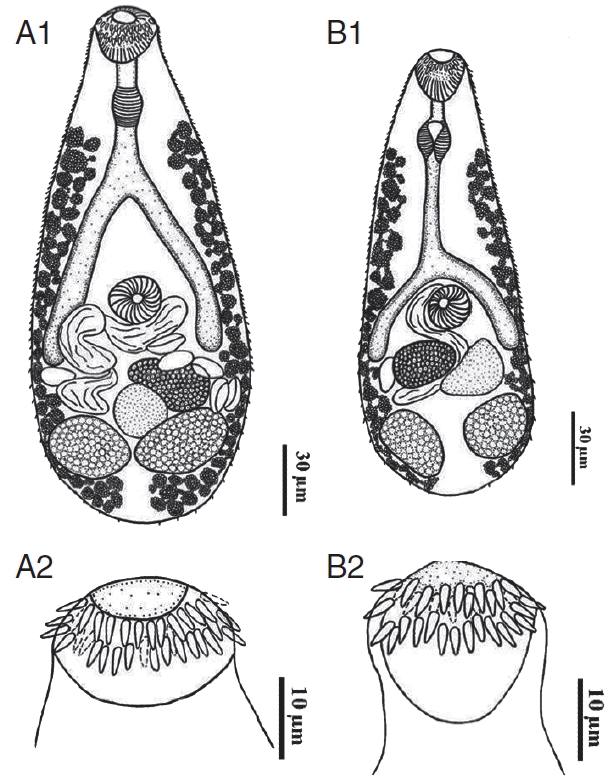
Fig. 2
Phylogenetic trees of C. formosanus originating from C. auratus in Chiang Mai, Thailand. (A) A phylogenetic tree analyzed by maximum-likelihood (ML) method using the MEGA program software version 6.0 with 1,000 bootstrap values. (B) Another tree analyzed by neighbor-joining (NJ) method using the MEGA program software version 6.0 with 1,000 bootstrap values.
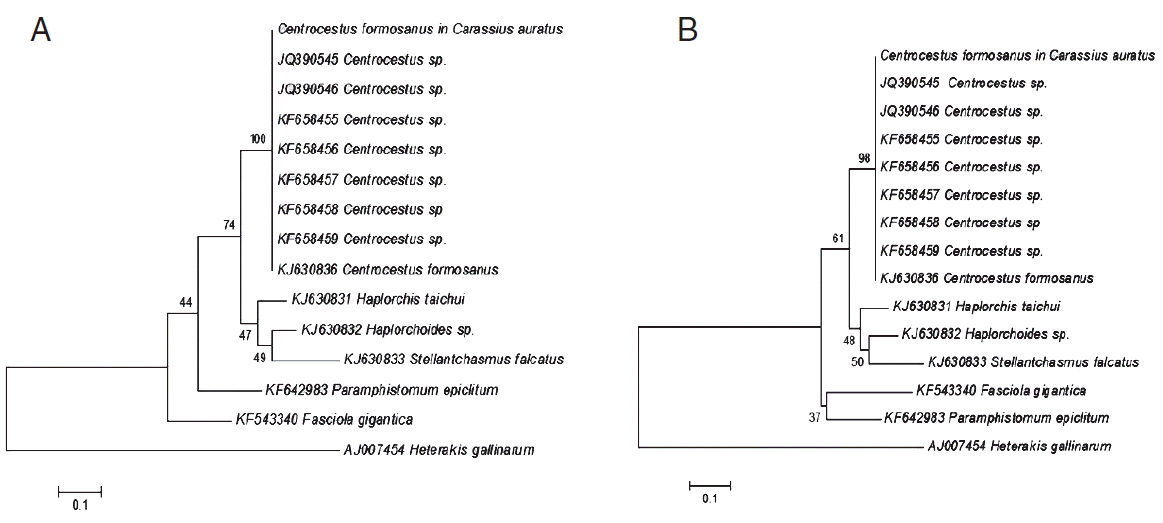
Table 1
Prevalence and intensity of infection with Centrocestus formosanus metacercariae in ornamental fish species from Chiang Mai, Thailand
|
Species of fish |
No. of fish examined |
No. of fish infected |
Prevalence (%) |
Intensitya
|
|
Carassius auratus (Goldfish) |
30 |
25 |
83.3 |
3.3 |
|
Cyprinus carpio (Koi) |
30 |
11 |
36.7 |
70.8 |
|
Poecilia latipinna (Sailfin Molly) |
30 |
5 |
16.7 |
1.4 |
|
Danio rerio (Zebrafish) |
30 |
6 |
20.0 |
5.2 |
|
Puntigrus tetrazona (Tiger barb) |
30 |
3 |
10.0 |
2.0 |
Table 2
Measurements (range and mean) of Centrocestus formosanus specimens originating from Carassius auratus (n=10) compared with 2 previous studies
Origin (fish)
Measurements (μm) |
Carassius auratus
Present study (Thailand) |
Puntius brevis
Han et al. (2008) (Lao PDR) |
Carassius auratus
Wongsawad et al. (2017) (Thailand) |
|
No. of circumoral spines |
34 |
32 (32–34) |
34 |
|
Body length |
465–552 (508.8) |
245–325 (286) |
600–750 (668) |
|
Body width |
176–224 (204.0) |
155–220 (192) |
200–290 (259) |
|
Oral sucker length |
48–58 (54.4) |
45–58 (52) |
60–80 (70.0) |
|
width |
62–66 (64.4) |
38–50 (43) |
52.5–82.5 (76.0) |
|
Prepharynx |
14–34 (22.4) |
- |
17.5–32.5 (26.5) |
|
Pharynx length |
36–46 (39.2) |
28–34 (32) |
45–57.5 (49.5) |
|
width |
30–40 (34.4) |
20–30 (26) |
37.5–50 (44.0) |
|
Esophagus |
26–50 (40.8) |
- |
32.5–62.5 (51.5) |
|
Ventral sucker length |
36–46 (42.8) |
45–55 (48) |
52.5–57.5 (55.5) |
|
width |
44–56 (51.2) |
33–45 (35) |
55–70 (65.5) |
|
Ovary length |
46–70 (56.0) |
50–80 (60) |
62.5–87.5 (76.5) |
|
width |
50–120 (81.2) |
34–46 (42) |
65–150 (105.5) |
|
Right Testis length |
60–80 (68.8) |
45–93 (65) |
57.5–92.5 (76.0) |
|
width |
94–110 (100.4) |
24–50 (38) |
100–137.5 (120.5) |
|
Left Testis length |
48–100 (68.0) |
55–88 (66) |
67.5–125 (87.0) |
|
width |
76–100 (86.0) |
30–63 (40) |
82.5–125 (103.5) |
|
Egg length |
32–38 (34.8) |
30–36 (34) |
40–47.5 (43.5) |
|
width |
20 |
15–19 (17) |
20–20 (20.0) |



