Cited By
Citations to this article as recorded by

Molecular Characterization and Analysis of Human Trichostrongylus Species in an Endemic Region of Iran Based on COX 1 Gene; A Cross‐Sectional Study
Sara Nemati, Hanieh Mohammad Rahimi, Meysam Sharifdini, Hamed Mirjalali
Health Science Reports.2025;[Epub]
CrossRef Gastrointestinal helminths in farmers and their ruminant livestock from the Coastal Savannah zone of Ghana
Sylvia Afriyie Squire, Rongchang Yang, Ian Robertson, Irene Ayi, Daniel Sai Squire, Una Ryan
Parasitology Research.2018; 117(10): 3183.
CrossRef Four clusters of Trichostrongylus infection diagnosed in a single center, in Italy
Dora Buonfrate, Andrea Angheben, Federico Gobbi, Manuela Mistretta, Monica Degani, Zeno Bisoffi
Prevalence and clinical aspects of human Trichostrongylus colubriformis infection in Lao PDR
Dorn Watthanakulpanich, Tiengkham Pongvongsa, Surapol Sanguankiat, Supaporn Nuamtanong, Wanna Maipanich, Tippayarat Yoonuan, Orawan Phuphisut, Boungnong Boupha, Kazuhiko Moji, Megumi Sato, Jitra Waikagul
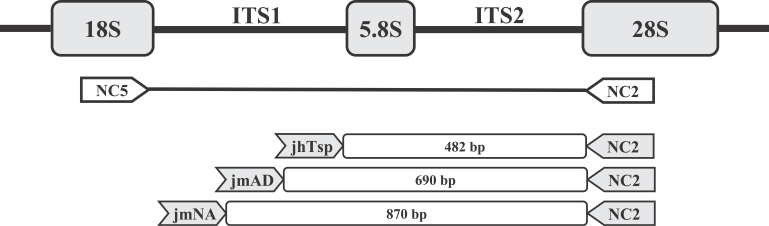
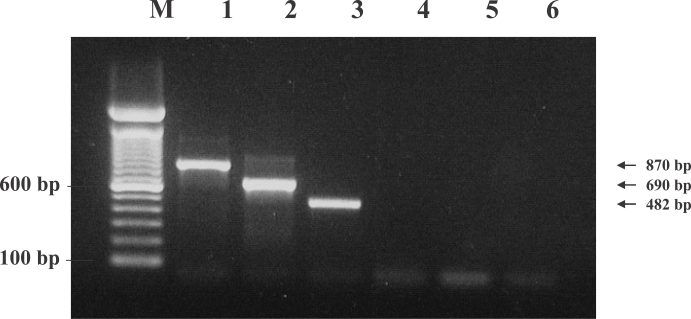
A multiplex restriction enzyme-PCR for unequivocal identification and differentiation of Trichostrongylus species in human samples
Azadeh Mizani, Pooria Gill, Ahmad Daryani, Shahabeddin Sarvi, Afsaneh Amouei, Ali Bakooie Katrimi, Eissa Soleymani, Siavash Mirshafiee, Sara Gholami, Seyed Abdollah Hosseini, Shirzad Gholami, Mohammad-Taghi Rahimi, Mohammad Bagher Hashemi-Soteh, Mehdi Sha
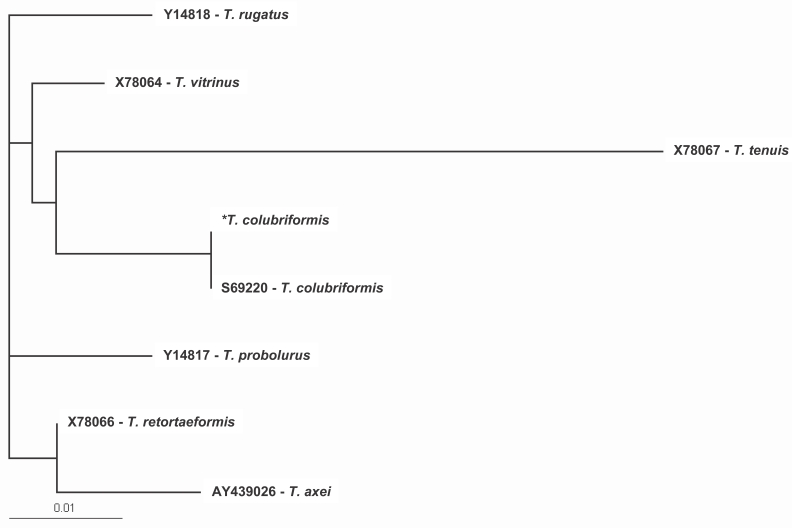
Molecular identification and phylogenetic analysis of human Trichostrongylus species from an endemic area of Iran
Meysam Sharifdini, Sedigheh Derakhshani, Safar Ali Alizadeh, Laleh Ghanbarzadeh, Hamed Mirjalali, Iraj Mobedi, Mehrzad Saraei
DNA technological progress toward advanced diagnostic tools to support human hookworm control
R.B. Gasser, C. Cantacessi, A. Loukas
Biotechnology Advances.2008; 26(1): 35.
CrossRef Key strongylid nematodes of animals — Impact of next-generation transcriptomics on systems biology and biotechnology
Cinzia Cantacessi, Bronwyn E. Campbell, Robin B. Gasser
Biotechnology Advances.2012; 30(3): 469.
CrossRef Rapid concentration and sensitive detection of hookworm ova from wastewater matrices using a real-time PCR method
P. Gyawali, J.P.S. Sidhu, W. Ahmed, P. Jagals, S. Toze
Experimental Parasitology.2015; 159: 5.
CrossRef Performance of microscopy compared to conventional PCR in identification of soil-transmitted helminth infections among antenatal women in a low-prevalence setting
Revathi Ulaganeethi, Vijaya Kumar Shettikothanuru Ramachandrappa, Nonika Rajkumari, Gowri Dorairajan, Ganesh Kumar Saya
Indian Journal of Medical Microbiology.2023; 46: 100427.
CrossRef Mitochondrial DNA reveals species composition and phylogenetic relationships of hookworms in northeastern Brazil
Kerla Joeline Lima Monteiro, Lauren Hubert Jaeger, Beatriz Coronato Nunes, Deiviane Aparecida Calegar, Elis Regina Chaves dos Reis, Polyanna Araújo Alves Bacelar, Jéssica Pereira dos Santos, Márcio Neves Bóia, Filipe Anibal Carvalho-Costa
Infection, Genetics and Evolution.2019; 68: 105.
CrossRef Molecular detection of Trichostrongylus species through PCR followed by high resolution melt analysis of ITS-2 rDNA sequences
Mohsen Arbabi, Hossein Hooshyar, Majid Lotfinia, Mohamad Ali Bakhshi
Molecular and Biochemical Parasitology.2020; 236: 111260.
CrossRef Copro-molecular identification of infections with hookworm eggs in rural Lao PDR
Megumi Sato, Surapol Sanguankiat, Tippayarat Yoonuan, Tiengkham Pongvongsa, Malaythong Keomoungkhoun, Inthava Phimmayoi, Boungnong Boupa, Kazuhiko Moji, Jitra Waikagul
Transactions of the Royal Society of Tropical Medicine and Hygiene.2010; 104(9): 617.
CrossRef Molecular diagnosis of infections and resistance in veterinary and human parasites
Peter W. Hunt
Veterinary Parasitology.2011; 180(1-2): 12.
CrossRef Trichostrongylosis: a zoonotic disease of small ruminants
A.H. Bhat, H. Tak, I.M. Malik, B.A. Ganai, N. Zehbi
Journal of Helminthology.2023;[Epub]
CrossRef Prevalence of human trichostrongyliasis in Iran: a systematic review and meta-analysis
Bahman Rahimi-Esboei, Maryam Pourhajibagher, Abbas Bahador
Reviews in Medical Microbiology.2022; 33(1): e16.
CrossRef Advances in molecular diagnosis of parasitic enteropathogens
Shane Byrne, Jennifer M.B. Robson
An Unusual Case of Hypereosinophilia and Abdominal Pain: An Outbreak ofTrichostrongylusImported From New Zealand
Emma C. Wall, Neha Bhatnagar, Julie Watson, Tom Doherty
Journal of Travel Medicine.2011; 18(1): 59.
CrossRef Repertory of eukaryotes (eukaryome) in the human gastrointestinal tract: taxonomy and detection methods
I. Hamad, D. Raoult, F. Bittar
Parasite Immunology.2016; 38(1): 12.
CrossRef Morphologic Characterization of Strongylida Larvae from Human and Swine Coprocultures in Rural Communities in the State of Piauí, Northeastern Brazil
Polyanna A. A. Bacelar, Kerla J. L. Monteiro, Jéssica P. dos Santos, Denilson de A. e Silva, Daniella N. Leal, Mayron M. Almeida, Brenda B. C. Evangelista, Francisco M. de Oliveira-Neto, Filipe A. Carvalho-Costa, Pedro P. Chieffi
Journal of Tropical Medicine.2022; 2022: 1.
CrossRef Development of a polymerase chain reaction applicable to rapid and sensitive detection ofClonorchis sinensiseggs in human stool samples
Pyo Yun Cho, Byoung-Kuk Na, Kyung Mi Choi, Jin Su Kim, Shin-Hyeong Cho, Won-Ja Lee, Sung-Bin Lim, Seok Ho Cha, Yun-Kyu Park, Jhang Ho Pak, Hyeong-Woo Lee, Sung-Jong Hong, Tong-Soo Kim
Pathogens and Global Health.2013; 107(5): 253.
CrossRef Zoonotic transmission of Teladorsagia circumcincta and Trichostrongylus species in Guilan province, northern Iran: molecular and morphological characterizations
Keyhan Ashrafi, Meysam Sharifdini, Zahra Heidari, Behnaz Rahmati, Eshrat Beigom Kia
BMC Infectious Diseases.2020;[Epub]
CrossRef Helminth and Intestinal Protozoa Infections, Multiparasitism and Risk Factors in Champasack Province, Lao People's Democratic Republic
Somphou Sayasone, Tippi K. Mak, Monely Vanmany, Oroth Rasphone, Penelope Vounatsou, Jürg Utzinger, Kongsap Akkhavong, Peter Odermatt, Jeffrey Michael Bethony
PLoS Neglected Tropical Diseases.2011; 5(4): e1037.
CrossRef Assessing the efficacy of albendazole against hookworm in Vietnam using quantitative PCR and sodium nitrate flotation
Clare E. F. Dyer, Naomi E. Clarke, Dinh Ng Nguyen, H. M. P. Dilrukshi Herath, Sze Fui Hii, Russell Pickford, Rebecca J. Traub, Susana Vaz Nery, Keke C. Fairfax
PLOS Neglected Tropical Diseases.2022; 16(10): e0010767.
CrossRef Infection dynamics of gastrointestinal helminths in sympatric non-human primates, livestock and wild ruminants in Kenya
Vincent Obanda, Ndichu Maingi, Gerald Muchemi, Chege J. Ng’ang’a, Samer Angelone, Elizabeth A. Archie, Emmanuel Serrano
PLOS ONE.2019; 14(6): e0217929.
CrossRef Improved molecular diagnostic tools for human hookworms
Robin B Gasser, Cinzia Cantacessi, Bronwyn E Campbell
Expert Review of Molecular Diagnostics.2009; 9(1): 17.
CrossRef High Prevalence of Haplorchis taichui, Phaneropsolus molenkampi, and Other Helminth Infections among People in Khammouane Province, Lao PDR
Jong-Yil Chai, Eun-Taek Han, Eun-Hee Shin, Woon-Mok Sohn, Tai-Soon Yong, Keeseon S. Eom, Duk-Young Min, Jin-Young Um, Min-Sung Park, Eui-Hyug Hoang, Bounlay Phommasack, Bounnaloth Insisiengmay, Soon-Hyung Lee, Han-Jong Rim
The Korean Journal of Parasitology.2009; 47(3): 243.
CrossRef Molecular Phylogenetics of Trichostrongylus Species (Nematoda: Trichostrongylidae) from Humans of Mazandaran Province, Iran
Meysam Sharifdini, Zahra Heidari, Zahra Hesari, Sajad Vatandoost, Eshrat Beigom Kia
The Korean Journal of Parasitology.2017; 55(3): 279.
CrossRef A Case for Using Genomics and a Bioinformatics Pipeline to Develop Sensitive and Species-Specific PCR-Based Diagnostics for Soil-Transmitted Helminths
Jessica R. Grant, Nils Pilotte, Steven A. Williams
Frontiers in Genetics.2019;[Epub]
CrossRef Prevalence and risk factors associated with gastrointestinal parasites in goats (Capra hircus) and sheep (Ovis aries) from three provinces of China
Weimin Cai, Cheng Cheng, Qianqian Feng, Yifei Ma, Enyu Hua, Shimin Jiang, Zhaofeng Hou, Dandan Liu, Anlong Yang, Darong Cheng, Jinjun Xu, Jianping Tao
Frontiers in Microbiology.2023;[Epub]
CrossRef Molecular Evidence of Trichostrongylus colubriformis and Trichostrongylus axei Infections in Humans from Thailand and Lao PDR
Kittisak Sawanyawisuth, Wanchai Maleewong, Issarapong Phosuk, Oranuch Sanpool, Tongjit Thanchomnang, Pewpan M. Intapan, Nimit Morakote, Penchom Janwan
The American Journal of Tropical Medicine and Hygiene.2013; 89(2): 376.
CrossRef Assessment of Real-Time Polymerase Chain Reaction for the Detection of Trichostrongylus spp. DNA from Human Fecal Samples
Francesca Perandin, Elena Pomari, Camilla Bonizzi, Manuela Mistretta, Fabio Formenti, Zeno Bisoffi
The American Journal of Tropical Medicine and Hygiene.2018; 98(3): 768.
CrossRef Human Trichostrongylus colubriformis Infection in a Rural Village in Laos
Boungnong Boupha, Kazuhiko Moji, Supaporn Nuamtanong, Tiengkham Pongvongsa, Inthava Phimmayoi, Jitra Waikagul, Vilayphone Phanhanan, Megumi Sato, Tippayarat Yoonuan, Surapol Sanguankiat
The American Journal of Tropical Medicine and Hygiene.2011; 84(1): 52.
CrossRef