Taenia saginata,
Taenia asiatica, and
Taenia solium are 3 tapeworm species that can cause zoonotic infections in humans. They have unique life cycles, with humans as the only definitive host [
1]. Beef is the infection source for
T. saginata, whereas
T. asiatica and
T. solium infections are contracted by pork. The distribution of human taeniases is worldwide, and
T. saginata is the most prevalent species [
2].
Little has been known about human
Taenia tapeworm infections in Cambodia with the exception of 2 studies reported in 2011 and 2014 [
3,
4]. According to the reports, fecal examinations of residents in Koh Kong Province [
3] and 18 nationwide provinces [
4] revealed the egg positive rate of
Taenia spp. to be 1.5% (43/2,824) and 0.4% (115/32,201), respectively. In Koh Kong Province, eggs of
Taenia spp. from 21 individuals were molecularly analyzed, and
T. saginata (n=19) and
T. solium (n=2) were identified by
cox1 sequencing and multiplex PCR [
3]. However, no other information is available regarding the species of
Taenia in other localities of Cambodia. In this study, we molecularly confirmed
T. saginata proglottids expelled from 2 residents after praziquantel treatment and purging in a northern part of Cambodia.
In May 2018, the Institute of Parasitic Diseases, Korea Association of Health Promotion (KAHP), in cooperation with the National Center for Parasitology, Entomology and Malaria Control, Cambodia, conducted a survey on intestinal parasitic infections in 2 northern provinces (5 villages each), Preah Vihear and Stung Treng (IRB no. 099NECHR, approved by National Ethics Committee for Health Research, Cambodia). A total of 1,156 fecal samples were collected from the residents and examined by the Kato-Katz thick smear method. The results revealed that the overall egg positive rate of
Taenia spp. was 2.4% (26/1,156); 1.7% (6/359) in Preah Vihear and 2.5% (20/797) in Stung Treng (
Table 1). A small village named Kampong Sangkae located in the northeastern part of Preah Vihear and bordered with Lao PDR showed a fairly high prevalence (3.4%, 3/89) of
Taenia spp. eggs. This village was selected for further analysis of
Taenia tapeworms in this study.
Two egg positive cases from this village were treated with 10–15 mg/kg praziquantel (Shinpoong Pharm. Co., Seoul, Korea) followed by purging with 30–40 g MgSO
4. The 2 tapeworm strobilae (1 was with scolex) (
Fig. 1A, B) discharged from the 2 cases were stored in 70% ethanol until morphological and molecular analyses. The scolex revealed 4 suckers but no recognizable rostellum nor hooklets (
Fig. 1B). Several proglottids from each of the 2 cases were fixed in 10% formalin and stained with Semichon’s acetocarmine, and morphologically observed using a light microscope. They were identified as either
T. saginata or
T. asiatica since they had >15 main uterine lateral branches filled with eggs (
Fig. 1C) [
5].
The genomic DNA of each tapeworm segment was extracted using the DNeasy Blood & Tissue kit (QIAGEN, Hilden, Germany) following the manufacturer’s instructions. PCR amplification and sequencing of mitochondrial cytochrome
c oxidase (
cox1, 1,620 bp), nuclear elongation factor-1α (
ef1, 1,090 bp), and nuclear ezrin-radixin-moesin (ERM)-like protein (
elp, 1,160 bp) genes were performed on each tapeworm sample with primers and conditions reported in a previous study [
6]. A phylogenetic tree of
cox1 gene constructed from the representative selection of sequences available in GenBank, using the maximum-likelihood method available in MEGA and employing Tamura-nei model of nucleotide substitution with 1,000 bootstrap replications, showed that our samples were both identical with
T. saginata (
Fig. 2). The sequence homology (
cox1) was 99.7% with
T. saginata and 94.8–95.2% with
T. asiatica. In addition, our samples contained the nuclear loci alleles
ef1C/ef1C and
elpC/elpC, which are homozygous for
T. saginata based on the interpretation using Sanger chromatogram analysis (
Table 2) [
6]. This demonstrates that our samples are not a hybrid between
T. saginata and
T. asiatica but ‘pure
T. saginata’.
Human taeniases are known to be endemic in Southeast Asia, including Myanmar [
7] and Lao PDR [
8]. In Cambodia, a few previous surveys reported low-grade prevalence of
Taenia spp. among the residents in several provinces [
3,
4]. Molecular studies on the species were reported in a paper in which the genomic DNA was extracted from eggs collected from the feces [
3]. In our study, we obtained gravid proglottids from 2 patients and observed that they had more than 15 main uterine lateral branches being consistent with
T. saginata or
T. asiatica but not with
T. solium. To determine whether they are
T. saginata or
T. asiatica, it was necessary to perform molecular analyses [
2].
Interestingly, hybrid descendants of
T. saginata and
T. asiatica were reported from humans in Thailand [
6] and Lao PDR [
8]. Therefore, we conducted molecular analyses of
cox1,
ef1, and
elp genes to rule out this hybrid issue. The
cox1 sequences revealed high homologies with
T. saginata but low homologies with
T. asiatica. The nuclear gene
ef1 is known to have 3 alleles (
ef1A,
ef1B, and
ef1C), and another nuclear gene
elp has 4 alleles (
elpA,
elpB,
elpC, and
elpD);
T. saginata has only 1
ef1 allele (
ef1C) and 3
elp alleles (
elpA,
elpC, and
elpD), whereas
T. asiatica has 2
ef1 alleles (
ef1A and
ef1B) and 2
elp alleles (
elpA and
elpB) [
6]. Our samples had
ef1C and
elpC alleles, and thus we could confirm them not a hybrid of the 2 species but pure
T. saginata.
The geographical distribution of 3 human
Taenia spp. is closely related to cultural characteristics of the people, which include the traditional food habit of consuming undercooked meat, including beef and pork, infected with viable metacestodes [
8]. Our study suggests the necessity of continued surveillance of human
Taenia tapeworm infections in Cambodia.
ACKNOWLEDGMENTS
We appreciate the staff of the National Center for Parasitology, Entomology and Malaria Control, Ministry of Health, Phnom Penh, Cambodia, and the Preah Vihear and Stung Treng Provincial Health Department, Cambodia for their help in this survey.
CONFLICTS OF INTEREST
CONFLICT OF INTEREST
We have no conflict of interest related to this work.
REFERENCES
1. Chai JY. Human taeniasis in the Republic of Korea: hidden or gone?
Korean J Parasitol 2013;51:9-17.



2. Eom KS, Rim HJ. Epidemiological understanding of
Taenia tapeworm infections with special reference to
Taenia asiatica in Korea.
Korean J Parasitol 2001;39:267-283.



3. Jeon HK, Yong TS, Sohn WM, Chai JY, Hong SJ, Han ET, Jeoung HG, Chhakda T, Sinuon M, Socheat D, Eom KS. Molecular identification of
Taenia tapeworms by
cox1 gene in Koh Kong, Cambodia.
Korean J Parasitol 2011;49:195-197.



4. Yong TS, Chai JY, Sohn WM, Eom KS, Jeoung HG, Hoang EH, Yoon CH, Jung BK, Lee SH, Sinuon M, Socheat D. Prevalence of intestinal helminths among inhabitants of Cambodia (2006–2011).
Korean J Parasitol 2014;52:661-666.



5. Mayta H, Talley A, Gilman RH, Jimenez J, Verastegui M, Ruiz M, Garcia HH, Ganzalez AE. Differentiating
Taenia solium and
Taenia saginata infections by simple hematoxylin-eosin staining and PCR-restriction enzyme analysis.
J Clin Microbiol 2000;38:133-137.



6. Okamoto M, Nakao M, Blair D, Anantaphrui MT, Waikagul J, Ito A. Evidence of hybridization between
Taenia saginata and
Taenia asiatica
.
Parasitol Int 2010;59:70-74.


7. Won EJ, Jung BK, Song H, Kim MS, Kim HS, Lee KH, Kim MJ, Shin MG, Shin JH, Suh SP, Hong SJ, Sohn WM, Htoon TT, Tin HH, Chai JY. Molecular diagnosis of
Taenia saginata tapeworm infection in 2 schoolchildren, Myanmar.
Emerg Infect Dis 2018;24:1156-1158.



8. Sato MO, Sato M, Yanagida T, Waikagul J, Pongvongsa T, Sako Y, Sanguankiat S, Yoonuan T, Kounnavang S, Kawai S, Ito A, Okamoto M, Moji K.
Taenia solium, Taenia saginata, Taenia asiatica, their hybrids and other helminthic infections occurring in a neglected tropical diseases’ highly endemic area in Lao PDR.
PLoS Negl Trop Dis 2018;12:e0006260.



Fig. 1
(A) A complete tapeworm strobila, including the scolex (inset), of Taenia saginata discharged from a resident in Kampong Sangkae village, Preah Vihear Province, Cambodia. Scale bar=2 cm. (B, C) Enlarged views of the scolex and a gravid proglottid taken with a stereomicroscope (Leica, Wetzlar, Germany). Morphological characteristics of the unarmed scolex with no distinct rostellum (B) and the high number (>15) of main uterine lateral branches (C) designated the tapeworm as either T. saginata or T. asiatica and far from T. solium. Scale bar=1 mm in (B) and 3 mm in (C).
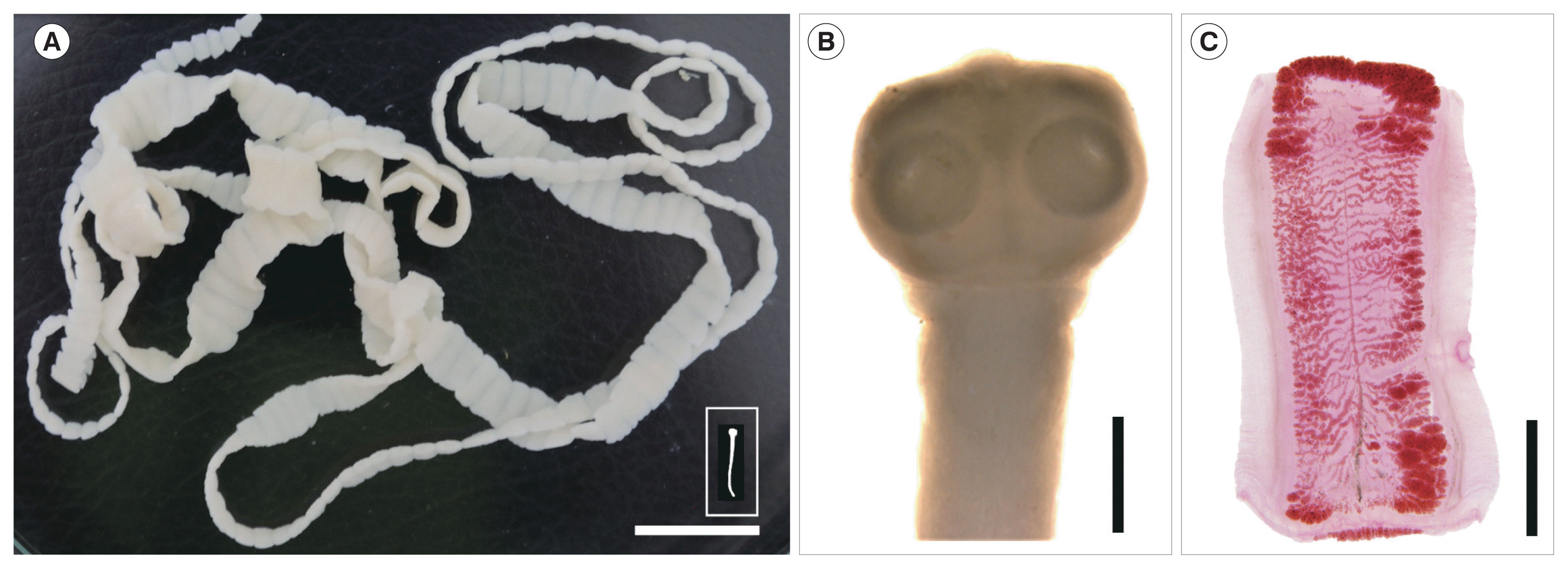
Fig. 2
A phylogenetic tree of 2 tapeworm samples obtained in this study in relation to tapeworm species drawn with cox1 DNA sequences in GenBank. Black dots indicate 2 samples identified in this study. Clonorchis sinensis was used as an outgroup. Scale bar indicates nucleotide substitutions per site.
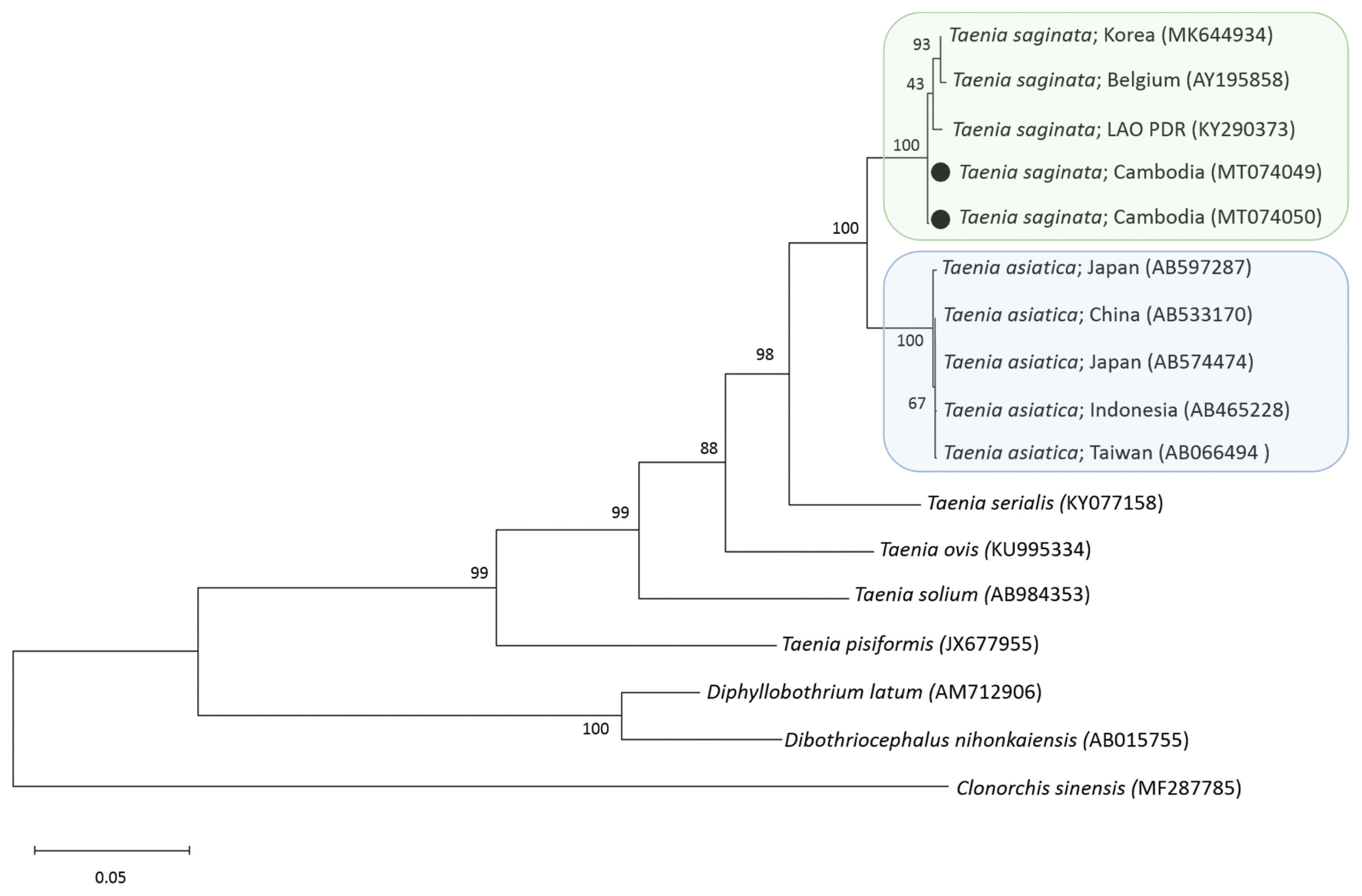
Table 1
Infection status of tapeworms in 2 northern provinces of Cambodia
|
Province |
Village |
No. of people examined |
No. of Taenia spp. egg positive cases (%) |
|
Preah Vihear |
Kampong Pou |
52 |
0 (0.0) |
|
Kampong Chey |
42 |
2 (4.8) |
|
Kampong Sangkaea
|
89 |
3 (3.4) |
|
Kampong Sralau |
74 |
0 (0.0) |
|
Kampong Sami |
50 |
1 (2.0) |
|
Subtotal |
359 |
6 (2.0) |
|
|
Stung Treng |
O’ Chay |
125 |
6 (4.8) |
|
Kanhchanh Tuek |
114 |
4 (3.5) |
|
Ti Team |
93 |
0 (0.0) |
|
Srae Russei |
204 |
4 (2.0) |
|
Peam Khes |
261 |
6 (2.3) |
|
Subtotal |
797 |
20 (2.5) |
|
|
Total |
|
1,156 |
26 (2.4) |
Table 2
Genotype of tapeworm samples from 2 patients
|
Patient no. |
mtDNA type (cox1) |
Genotypea at ef1 locus |
Genotypea at elp locus |
|
1 |
T. saginata
|
ef1C/ef1C (T. saginata) |
elpC/elpC (T. saginata) |
|
2 |
T. saginata
|
ef1C/ef1C (T. saginata) |
elpC/elpC (T. saginata) |



