Cited By
Citations to this article as recorded by

Genotypes of Blastocystis sp. among elderly health checkup people in South Korea with a questionnaire on risk factors
Taehee Chang, Bong-Kwang Jung, Hyejoo Shin, Sooji Hong, Seungwan Ryoo, Jeonggyu Lee, Seihee Han, Sun Min Park, Min-Suk Rhee, Heejin Kimm, Sun Ha Jee, Jong-Yil Chai
Parasitology Research.2021; 120(9): 3297.
CrossRef Prevalence of Blastocystis and Dientamoeba fragilis in diarrheal patients in Corum, Türkiye
Ayşe Semra Gureser, Djursun Karasartova, Fakhiriddin Sarzhanov, Nezahat Kosar, Aysegul Taylan-Ozkan, Funda Dogruman-Al
Parasitology Research.2023; 122(12): 2977.
CrossRef Molecular identification and subtyping of Blastocystis sp. in hospital patients in Central China
Junqiang Li, Haiju Dong, Md Robiul Karim, Xianli Yang, Liqin Chao, Shuai Liu, Hairong Song, Longxian Zhang
European Journal of Protistology.2021; 79: 125796.
CrossRef First Report ofBlastocystisspp. Infection in Pet Birds in Henan Province, Central China
Changwei Su, Xuefang Mei, Lai Wei, Fuqiang Zhang, Jiawen Wang, Yuan Chang, Mingyong Wang, Xiaowei Tian, Zhenchao Zhang, Xiangrui Li, Shuai Wang
Vector-Borne and Zoonotic Diseases.2022; 22(7): 370.
CrossRef Effect of antibiotic administration on Blastocystis persistence and gut microbiome–metabolome dynamics in an irritable bowel syndrome longitudinal case study
Jamie M. Newton, William J.S. Edwards, Gary S. Thompson, Eleni Gentekaki, Anastasios D. Tsaousis
Access Microbiology
.2025;[Epub]
CrossRef Prevalence and molecular subtyping of Blastocystis sp. in rabbits in Henan, Central China
Changwei Su, Xuefang Mei, Xia Feng, Fuqiang Zhang, Pei Wang, Bo He, Fuyang Xu, Zishan Yang, Xiaowei Tian, Zhenchao Zhang, Xiangrui Li, Shuai Wang
Folia Parasitologica.2022;[Epub]
CrossRef The Coexistence of Blastocystis spp. in Humans, Animals and Environmental Sources from 2010–2021 in Asia
Adedolapo Aminat Rauff-Adedotun, Farah Haziqah Meor Termizi, Nurshafarina Shaari, Ii Li Lee
Metabolic Fluctuations in the Human Stool Obtained from Blastocystis Carriers and Non-Carriers
Emma L. Betts, Jamie M. Newton, Gary S. Thompson, Fakhriddin Sarzhanov, Vasana Jinatham, Moon-Ju Kim, Siam Popluechai, Funda Dogruman-Al, Eun-Jeong Won, Eleni Gentekaki, Anastasios D. Tsaousis
Gut Microbiome Profiles in Colonizations with the Enteric Protozoa Blastocystis in Korean Populations
Moon-Ju Kim, Yu Jeong Lee, Tae-Jong Kim, Eun Jeong Won
Microorganisms.2021; 10(1): 34.
CrossRef Molecular Prevalence of Blastocystis sp. from Patients with Diarrhea in the Republic of Korea
Ji-Young Kwon, Jong-Hoon Choi, Hee-Il Lee, Jung-Won Ju, Myoung-Ro Lee
Microorganisms.2024; 12(3): 523.
CrossRef Subtype Distribution of Blastocystis spp. in Patients with Gastrointestinal Symptoms in Northern Spain
Cristina Matovelle, Joaquín Quílez, María Teresa Tejedor, Antonio Beltrán, Patricia Chueca, Luis Vicente Monteagudo
Microorganisms.2024; 12(6): 1084.
CrossRef Genetic Analysis of Zoonotic Gastrointestinal Protozoa and Microsporidia in Shelter Cats in South Korea
Dongmi Kwak, Min-Goo Seo
Prevalence and Associated Factors of Blastocystis sp. Infection in Patients with Gastrointestinal Symptoms in Spain: A Case-Control Study
Cristina Matovelle, María Teresa Tejedor, Luis Vicente Monteagudo, Antonio Beltrán, Joaquín Quílez
Tropical Medicine and Infectious Disease.2022; 7(9): 226.
CrossRef Systematic Review and Meta-Analysis: Epidemiology of Human Blastocystis spp. Infection in Malaysia
Vinoth Kumarasamy, Arutchelvan Rajamanikam, Deepa Anbazhagan, Wahib Mohammed Atroosh, Meram Azzani, Vetriselvan Subramaniyan, Syamsa Rizal Abdullah
Tropical Medicine and Infectious Disease.2023; 8(8): 415.
CrossRef
Abstract
Blastocystis has recently been recognized as the most common eukaryotic microbe of the human gut. We investigated the prevalence of Blastocystis and their subtypes in diarrheal and non-diarrheal groups and the associated clinical parameters. A total of 324 stool samples were obtained from 196 diarrheal and 128 non-diarrheal subjects. Blastocystis subtypes were determined by sequencing the small subunit ribosomal DNA (SSU rRNA) gene. Demographic, clinical and laboratory data were collected and analyzed by diarrhea and Blastocystis status. The overall rate of Blastocystis positivity was 9.0% (29/324) but was significantly higher in the non-diarrheal group (18.0% vs. 3.1%, P<0.0001). Of the 6 Blastocystis-positive diarrheal patients, 3 (50.0%), none (0.0%), 2 (33.3%), and 1 (16.7%) were infected with subtypes ST1, ST2, ST3, and multiple subtypes, respectively. Of the 23 Blastocystis-positive non-diarrheal patients, 4 (17.4%), 1 (4.3%), and 18 (78.3%) were infected with subtypes ST1, ST2, and ST3, respectively. Blastocystis was less common in the diarrheal than the non-diarrheal group (odds ratio, 0.144; 95% confidence interval, 0.057–0.365, P<0.001). Of the 3 subtypes, ST3 was more frequently observed in the non-diarrheal than diarrheal group (78.3% vs. 33.3%, P=0.0341). Collectively, Blastocystis was found in both the diarrheal and non-diarrheal groups and ST3 was the most common subtype in Korea.
Key words: Blastocystis, subtype, diarrhea, Korea
Blastocystis has recently been recognized as the most prevalent eukaryotic microbe in the human gut [
1], occurring worldwide in both healthy and symptomatic humans and other animals.
Blastocystis is thought to be transmitted via the fecal–oral route and in cyst form [
2]. Recently, PCR-based approaches using feces directly or after culture of fecal specimens have been widely used to diagnose
Blastocystis infection [
3]. Based on small subunit ribosomal DNA (SSU rRNA) gene analysis, this genus comprises at least 17 subtypes (STs) [
4]. In human, ST1–ST4 probably account for more than 90% of carriage; the other subtypes are ST5–ST9 [
5]. Although
Blastocystis is of great scientific interest, neither its biology nor pathophysiology has been well-explored. Since no direct evidence indicates that
Blastocystis causes diarrhea, this point also remains controversial. Only 3 Korean studies on
Blastocystis have been published [
6–
8], all in animals. Here, we investigated the prevalence of
Blastocystis and its subtypes, and the associated clinical parameters, in diarrheal and asymptomatic Korean groups.
A total of 324 stool samples were obtained from 196 diarrheal and 128 non-diarrheal subjects who underwent general checkups at Chonnam National University Hospital and Chonnam National University Hwasun Hospital, from February 2016 to October 2018. Fecal samples were collected in accordance with the guidelines of, and with the approval of, the Institutional Review Board of Chonnam National University Hospital (approval no. IRB CNUH-2015-052). We recorded age, sex, white blood cell count (WBC,×10
3/μl), and differential counts, red blood cell count (RBC,×10
6/μl), hemoglobin (Hgb, g/dl), platelet count (PLT), total protein (g/dl), albumin (g/dl), alkaline phosphatase (U/L), AST (U/L), ALT (U/L), BUN (mg/dl), creatinine (mg/dl), lactate dehydrogenase (U/L), CRP (mg/dl), and stool occult blood test data. If necessary, stool culture or multiplex PCR panel evaluation was scheduled to determine pathogens causative of diarrhea. Laboratory findings and stool culture data were analyzed by diarrhea and
Blastocystis status. DNA was extracted using the Cica Geneus® DNA Prep Kit (Kanto Chemical, Tokyo, Japan) following the manufacturer’s instructions.
Blastocystis was detected based on the SSU rRNA gene using the Blast-505–532 (5′–GGA GGT AGT GAC AAT AAA TC–3′) and Blast-998–1017 (5′–TGC TTT CGC ACT TGT TCA TC–3′) primers [
9]. Each tube contained 8.5 μl PCR primer solution (1 μl each of Blast-505–532 and Blast-998–1017 (each 25 pmol)), 36.5 μl distilled water, and 5 μl template DNA. All PCR amplifications were performed using the TaKaRa PCR Thermal Cycler Dice Gradient (TaKaRa, Tokyo, Japan). Initial denaturation at 94°C for 3 min was followed by 30 cycles of 59°C for 30 sec and 72°C for 60 sec, and a final extension at 72°C for 5 min. The PCR products were analyzed by 1.5% (w/v) agarose gel electrophoresis with ethidium bromide staining and then sent to Macrogen (Seoul, Korea) for direct DNA sequencing. Phylogenetic analysis was performed by reference to database
Blastocystis SSU rRNA genes, and a phylogenetic tree was constructed using Geneious Prime (Biomatters Ltd, Auckland, New Zealand). Phylogenetic inferences were derived using a pair-group method featuring arithmetic average clustering with 1,000 bootstrap replications. Student’s t-test was used to compare continuous variables (age and laboratory parameters). The chi-squared or Fisher’s exact test was employed to determine the distributions of categorical variables (sex and the statuses of stool occult blood, diarrhea, and
Blastocystis). The likelihood-ratio chi-squared test was employed to calculate odds ratios (ORs) for
Blastocystis positivity by subtype. All statistical analyses were performed using SPSS ver. 25.0 software (SPSS Inc., Chicago, Illinois, USA). A
P-value <0.05 was considered to indicate significance.
Of the 324 samples, 29 (9.0%) including 6 diarrheal and 23 non-diarrheal samples were positive for
Blastocystis, with a significant difference between the 2 groups (
P<0.001) (
Table 1). Thus,
Blastocystis may not necessarily cause diarrhea.
Blastocystis has previously been found in both symptomatic and asymptomatic patients [
10–
12]. It has been suggested that intra-subtype variation at the SSU rRNA gene level might affect the presenting symptoms of patients with identical
Blastocystis subtypes [
13]. We found subtypes ST1, ST2, ST3, and multiple infections in 3 (50.0%), 0 (0.0%), 2 (33.3%), and 1 (16.7%) patient in the diarrheal group and in 4 (17.4%), 1 (4.3%), 18 (78.3%), and 0 (0.0%) patient the non-diarrheal group. Of the 3 subtypes, ST3 was more common in the non-diarrheal group (78.3 vs. 33.3%,
P=0.0341). ST1 seemed to be frequently found in diarrheal group compared to non-diarrheal group, but no statistical significance (diarrheal group vs. non-diarrheal group, 3/6 vs. 4/23,
P=0.0964). Further study may be necessary to determine potential correlation of certain subtypes and symptoms. We did not detect ST4, in agreement with previous studies suggesting that this ST was rare in Asia and the Middle East [
14,
15]. When we analyzed the distribution of STs, a total of 26 sequenced samples (GenBank accession no. MT186203-MT186218 enrolled in this study) were closely related to the sequences of each known human STs (
Fig. 1). All but 2 ST3 sequences clustered together with sequences from the other countries such as Germany (MK801366, MK801409), Singapore (KX618192), Poland (MN918265), Mexico (MK874780, KU147402), South Korea (MT093452), Iran (MH049544, LC414153) and Thailand (JX305884), respectively. Notably, 2 ST3 sequences (MT186204, MT186206) from the diarrheal group were somewhat distant from the others; ST3 thus exhibited intra-genetic variation. All sequences lay distant from those of ST4 (KX351997, KP284173), ST5 (MT094303, KM438216), ST6 (MG011651, KP284174), and ST7 (JN003686), respectively. We also analyzed several laboratory parameters by
Blastocystis presence or symptoms. The white and red blood cell counts, hemoglobin level, percentages of lymphocytes, monocytes, eosinophils, and basophils, and the levels of total protein, albumin, BUN, lactate dehydrogenase, gamma-glutamyl transpeptidase, and C-reactive protein, and the rate of positive results on stool occult blood tests differed significantly between the diarrheal and non-diarrheal groups. However, only the creatinine level differed significantly between the
Blastocystis-positive and -negative groups (3.6 vs. 1.1 mg/dl,
P=0.001) (
Table 2). Of the 196 diarrheal patients, 54 exhibited other causative pathogens (
Clostroides difficile with or without other pathogens (23),
Campylobacter spp. with or without other pathogens (15),
Salmonella spp. (9),
Citrobacter freundii (1),
Cryptosporidium spp. (1),
Enterococcus faecalis (1), ETEC LT/ST, STEC stx1/sb2 (1), Norovirus GI/GII (1),
Giardia spp. (1) and
Shigella spp. (1)). Of the 29
Blastocystis-positive patients, all but 2 (one with
Campylobacter sp. and one with
Giardia sp.) were negative for other possibly causative pathogens. Overall, our data support the suggestion that
Blastocystis may not be pathogenic.
This is the first report of Blastocystis infections in Koreans. Blastocystis was found in both the diarrheal and non-diarrheal groups, but the subtype prevalence differed between the groups. Any role played by Blastocystis in human health and disease should be explored further.
Fig. 1
Phylogenetic tree of 26 sequences of Blastocystis SSU rRNA gene compared to database Blastocystis SSU rRNA genes. All of sequences of this study were enrolled to GenBank database (GenBank accession no. MT186203-MT186218), which were indicated in bold.
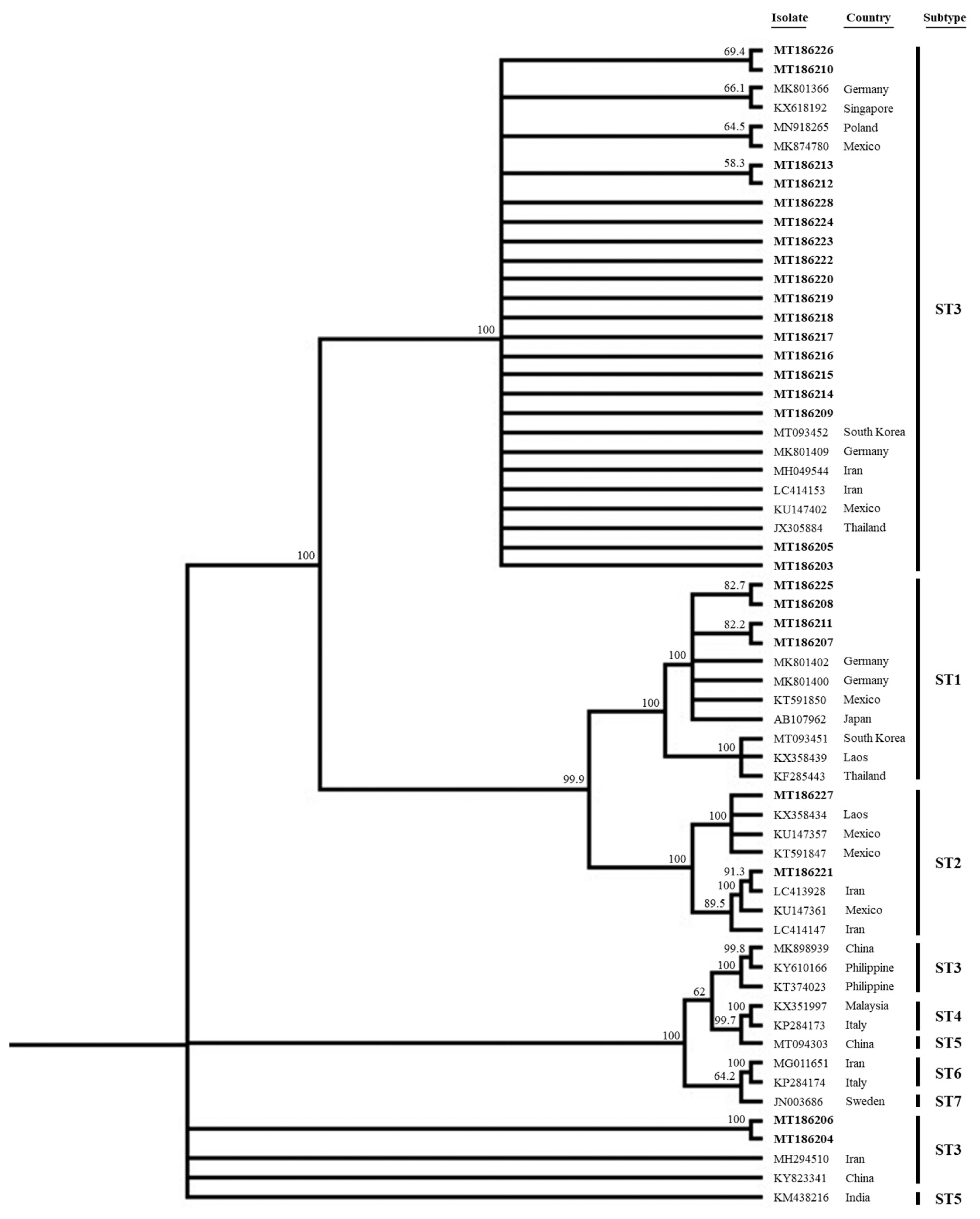
Table 1
Characteristic of laboratory findings according to the diarrheal symptoms
|
Clinical parametersa
|
Laboratory characteristics of |
P-value |
|
Diarrheal group (n=196) |
Healthy control (n=128) |
|
Age (yr) |
60.8±17.78 |
62.6±11.37 |
0.318 |
|
WBC count (×103/μl) |
9±5.61 |
5.6±1.43 |
<0.001 |
|
RBC count (×106/μl) |
3.6±0.66 |
4.8±0.43 |
<0.001 |
|
Hemoglobin (g/dl) |
10.9±2.22 |
14.1±1.33 |
<0.001 |
|
Platelet count (×103/μl) |
219.6±136.82 |
252.3±54.3 |
0.13 |
|
Lymphocytes (%) |
15.8±11.27 |
34.9±8.01 |
<0.001 |
|
Monocytes (%) |
9.3±7.54 |
6.8±2.18 |
0.034 |
|
Neutrophils, (%) |
73.1±14.94 |
66.1±73.8 |
0.224 |
|
Eosinophils (%) |
1.9±2.21 |
2.8±2.69 |
0.015 |
|
Basophils (%) |
0.4±0.38 |
0.7±0.27 |
<0.001 |
|
Total protein (g/dL) |
6±0.99 |
7.5±0.48 |
<0.001 |
|
Albumin (g/dL) |
3.3±0.75 |
4.4±0.3 |
<0.001 |
|
ALP (U/L) |
114.4±134.06 |
75.7±22.59 |
0.058 |
|
AST (U/L) |
36.1±38.3 |
29±12.01 |
0.067 |
|
ALT (U/L) |
28.4±29.73 |
27.7±18.33 |
0.829 |
|
Total Bilirubin (mg/dL) |
1.2±2.11 |
0.8±0.33 |
0.348 |
|
BUN (mg/dl) |
17.6±14.35 |
12.1±4.89 |
0.011 |
|
Creatinine (mg/dl) |
1.2±3.36 |
1.3±3.51 |
0.812 |
|
Lactate dehydrogenase (U/L) |
618.9±450.65 |
141.5±94.76 |
<0.001 |
|
r-GTP (IU/L) |
109±147.21 |
31.5±28.56 |
<0.001 |
|
CRP (mg/dl) |
7.8±7.69 |
0.1±0.13 |
<0.001 |
|
Sex (male) |
116/196 (59.2) |
54/128 (42.2) |
0.003 |
|
Presence of abdominal pain No. (%) |
92/196 (46.9) |
4/128 (3.1) |
<0.001 |
|
Positive for stool occult blood No. (%) |
9/23 (39.1) |
0/86 (0.0) |
<0.001 |
|
Positive for Blastocystis, No. (%) |
6/196 (3.1) |
23/128 (18.0) |
<0.001 |
|
Blastocystis subtype No. (%) |
|
ST1 |
3/6 (50.0) |
4/23 (17.4) |
0.0964 |
|
ST2 |
0/6 (0.0) |
1/23 (4.3) |
|
|
ST3 |
2/6 (33.3) |
18/23 (78.3) |
0.0341 |
|
Multi band |
1/6 (16.7) |
0/23 (0.0) |
|
Table 2
Characteristic of laboratory findings according to the presence of Blastocystis
|
Clinical parametersa
|
Laboratory characteristics |
P-value |
|
Blastocystis-negative (n=295) |
Blastocystis-positive (n=29) |
|
Age (yr) |
61.4±15.94 |
62.6±11.48 |
0.692 |
|
WBC count (×103/μl) |
8.5±5.29 |
6.5±4.75 |
0.234 |
|
RBC count (×106/μl) |
3.8±0.76 |
4.1±0.9 |
0.301 |
|
Hemoglobin (g/dl) |
11.9±2.48 |
12.7±2.2 |
0.147 |
|
Platelet count (×103/μl) |
226.6±128.34 |
197.7±82.64 |
0.482 |
|
Lymphocytes (%) |
19.1±13.11 |
23.9±11.13 |
0.254 |
|
Monocytes (%) |
8.8±6.99 |
8.9±5.8 |
0.965 |
|
Neutrophils (%) |
72.1±34.9 |
64.4±13.94 |
0.488 |
|
Eosinophils (%) |
2±2.35 |
2.2±2.1 |
0.808 |
|
Basophils (%) |
0.4±0.39 |
0.5±0.38 |
0.495 |
|
Total protein (g/dl) |
6.3±1.09 |
6.5±1.07 |
0.606 |
|
Albumin (g/dl) |
3.5±0.82 |
3.9±0.56 |
0.083 |
|
ALP (U/L) |
107.5±121.95 |
64.9±14.12 |
0.357 |
|
AST (U/L) |
34.3±32.85 |
24.8±7.42 |
0.177 |
|
ALT (U/L) |
28.5±27.09 |
23.5±10.74 |
0.387 |
|
Total Bilirubin (mg/dl) |
1.1±1.93 |
0.7±0.2 |
0.515 |
|
BUN (mg/dl) |
16.7±13.25 |
13±12.89 |
0.299 |
|
Creatinine (mg/dl) |
1.1±2.82 |
3.6±7.37 |
0.001 |
|
Lactate dehydrogenase (U/L) |
499.6±446.27 |
225.9±199.58 |
0.109 |
|
r-GTP (IU/L) |
70.8±112.65 |
25.6±17.51 |
0.137 |
|
CRP (mg/dl) |
6.8±7.69 |
5.5±5.94 |
0.613 |
|
Sex male No. (%) |
159/295 (53.9) |
11/29 (37.9%) |
0.12 |
|
Presence of abdominal pain No. (%) |
93/295 (31.5) |
3/29 (10.3%) |
0.018 |
|
Positive for stool occult blood No. (%) |
9/95 (9.5) |
0/14 (0.0%) |
0.601 |
|
Positive for Diarrhea No. (%) |
190/295 (64.4) |
6/29 (20.7%) |
< 0.001 |
|
Other pathogens No. (%) |
52/295 (17.6)b
|
2/29 (6.9%)c
|
|




