INTRODUCTION
The 2 genera,
Toxocara and
Neoascaris, of the superfamily Ascaridoidea have a close morphological relationship, the genetic and morphological differentiations between 2 genera incompletely remain yet [
1]. The subfamily Toxocarinae is morphologically characterized by a ventriculus without appendices and the absence of gubernaculum. Additionally, the members in the genus
Toxocara does not have the intestinal cecum and interlabia [
2]. And then in the species identification of the genus
Toxocara, some morphological characteristics, i.e., the shape of cervical alae in the cross-sectional samples, the spicule length and lip structure act as the differential keys [
3]. According to the morphological characteristics of the genus
Toxocara reported by Warren [
1], this nematode group with genus
Toxascaris are differentiated from genus
Ascaris by the arrow-shape head, and they are separated each other by the egg shape. The eggs of
Toxocara spp. have corrugated shells but those of
Toxascaris have smooth shells.
More than 28 species including some synonyms have been reported in the genus
Toxocara from a variety of host animals [
3–
13]. Most of these parasitize in terrestrial mammals except for
T. pearsel, which is a parasite of snapping shrimp,
Synalpheus brooksi [
3,
14]. At least 5 species, i.e.,
T. canis from canids,
T. cati from felids,
T. vitulorum from ruminants,
T. pteropodis from bats and
T. malaysiensis from cats, are known to be the zoonotic agents [
4,
5]. Another species of
Toxocara have been reported in bats (
T. cynonycterides), canids (
T. marginata), carnivores (
T. alienata,
T. anakumae,
T. herpestum,
T. paradoxura,
T. sprenti,
T. suricattae,
T. tanuki and
T. vajrasthirae), elephants (
T. elephantis), felids (
T. canarisi,
T. genettae and
T. lyncis), hippopotamus (
T. hippopotami and
T. vincenti), ruminant (
T. manzadiensis and
T. warren), and rodents (
T. apodemi,
T. indica and
T. mackerrasae) respectively.
Among the toxocarid nematodes,
T. canis and
T. cati are most commonly found from the intestines of dogs and cats worldwide [
15,
16]. A variety of
Toxocara species is commonly found in livestock, but their available scientific informations are not enough, especially about a toxocarid from wild field mice,
Apodemus agrarius.
Toxocara apodemi was first reported from the intestine of the wood mouse,
Apodemus peninsulae from the Central National Forest, Bupyeong-ri, Namyangju-si, Gyeonggi-do, Korea [
13]. This nematode was reported as a new species of the genus
Neoascaris, and named as
Neoascaris apodemi by Olsen in 1957. Later, this species of nematode was found in the small intestine of striped field mice in China and emended as
T. apodemi [
6], although it was more or less small than
N. apodemi [
13].
Traditionally,
Toxocara spp. have been identified and classified based on their morphological features and host species [
1–
3]. Also, DNA sequencing is recently used with identification for
Toxocara species [
17]. Nevertheless, there is no reports for identification of
T. apodemi by scanning electron microscopy (SEM) study and genetic information. The present study provides the first SEM data about the morphology of
T. apodemi and its genetic characterization using 18S rRNA analysis.
DISCUSSION
The subfamily Toxocarinae is characterized by the presence of a ventriculus without appendices and the absence of interlabia and gubernaculum [
2]. In this study, the nematodes did not have intestinal cecum, interlabia, appendices of ventriculus, or gubernaculum. Also, the parasites were isolated in
A. agrarius captured from 3 areas (Uljin-gun and Yongduk-gun in Geongbuk-do and Iksan-city in Chonbuk-do). The
Toxocara spp. of Muridae include
T. apodemi and
T. mackerrasae [
1,
13]. They differ in their selection of hosts; the
T. mackerrasae infects
Rattus fuscipes and
Hydromys chrysogaster, while
T. apodemi infects
A. peninsulae and
A. agrarius.
T. apodemi was reported to infect
A. peninsulae and
A. agrarius in China [
6,
9,
13]. Currently, in Korea,
T. apodemi infects
A. peninsulae and
A. agrarius.
The diagnostic keys for
Toxocara created by Warren [
1] indicate that the external prolongations of labial pulp in
T. apodemi are symmetrical and rounded at the anterior border. The terminal spike on the tail of the female is present in
T. apodemi but not in
T. mackerrasae. The spicules of
T. apodemi are 0.55 mm long in males 35.0 mm and 0.65 mm long in 35 to 36 mm long. The measurements of spicules of other species, including
T. mackerrasae (0.65 mm),
T. tanuki (3.0–3.5 mm),
T. cati (1.71–1.90 mm),
T. canis (0.91–0.97 mm),
T. pteropodis (0.58–0.73 mm),
T. vincenti (0.68 mm),
T. paradoxum (2.71–3.12 mm),
T. pearsei (1.2–1.32 mm),
T. malysiensis (right 0.46–0.68 mm and left 0.48–0.76 mm),
T. lyncis (1.3–1.8 mm), and
T. hippopotami (0.83–1.01 mm) have been reported [
1,
3,
8,
11]. In this study, the spicules were subequal and measured 0.45–0.53 mm long. The spicules were smaller than that of other species but closer to
T. apodemi (0.45–0.54 mm) [
13].
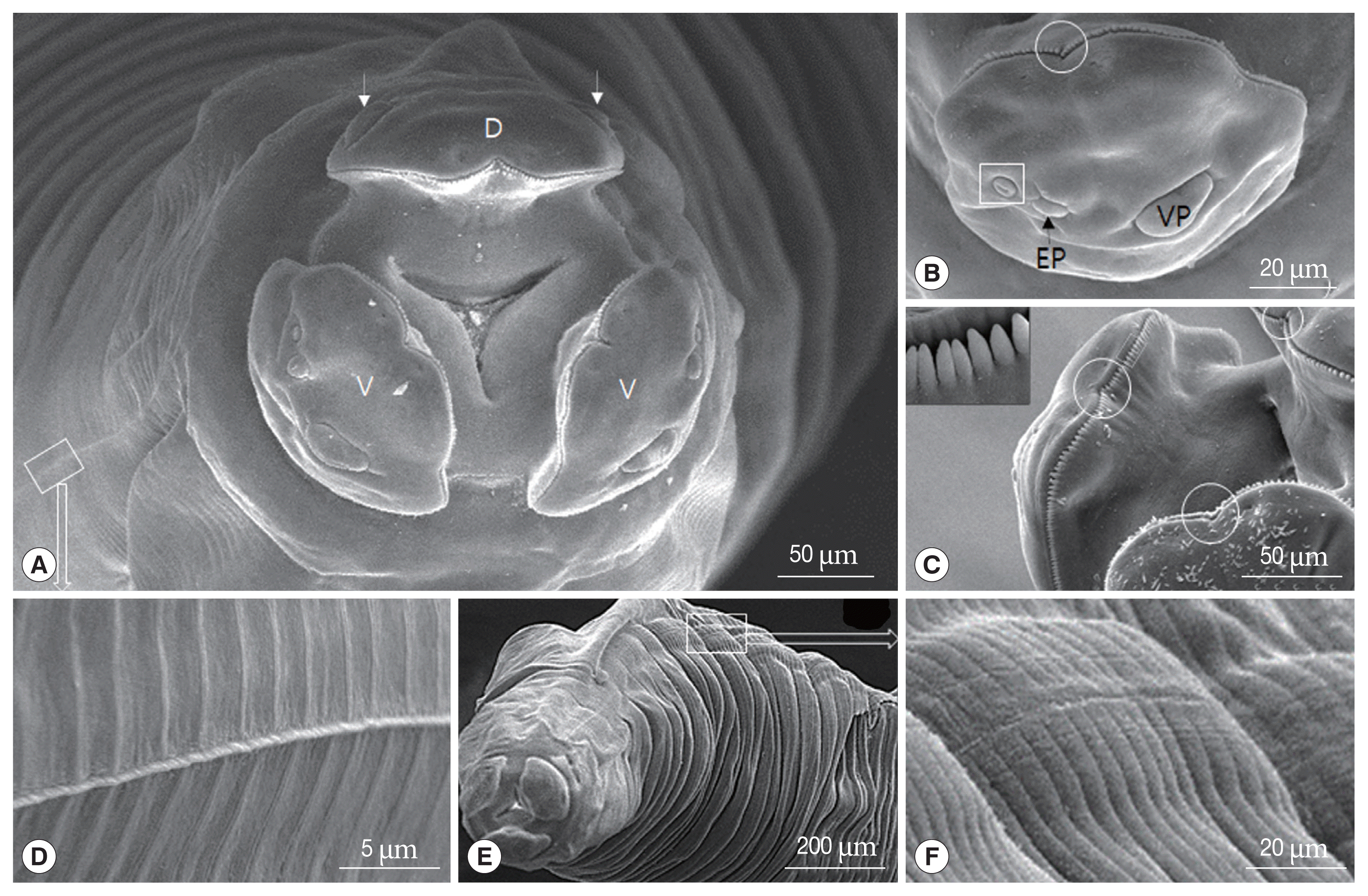
The 3 labia in this study were present as in ascaridoid nematodes and the denticles had a consistent distribution and a triangular outline of labium; however,
T. malaysiensis has a deep median notch lined with denticles of the dentigerous ridge, which is absent in the most toxocarids, such as
T. canis,
T. cati, and
T. tanuki [
1,
11]. In this study, the specimen also has strong denticles on the dentigerous ridge and a superficial median notch on each lip.
Gibbons et al. [
3] reported a new species,
T. malaysiensis, based on the following features: presence of the esophagus with a ventriculus without an appendix, the absence of an intestinal cecum, prominent dentigerous ridges on each of the 3 lips, a V-shaped supporting bar in the cervical alae, male with sub-equal, alate spicules, female with vulva opening in the anterior half of the body, and sub-globular eggs with a thick pitted shell.
Toxocara spp., including
T. canis,
T. cati,
T. hippopotami,
T. lyncis,
T. malaysiensis,
T. pteropodis,
T. tanuki, and
T. vincenti, have cervical alae [
3,
6,
8], while
T. indica and
T. apodemi do not have cervical alae [
12,
13].
T. indica differs from all the known species of the genus
Toxocara in having subequal spicules and the absence of cervical alae, except
T. alienta and
T. apodemi.
T. indica resembles
T. alienate in the absence of cervical alae [
12]. Asakawa et al. and Olsen [
6,
13] reported that
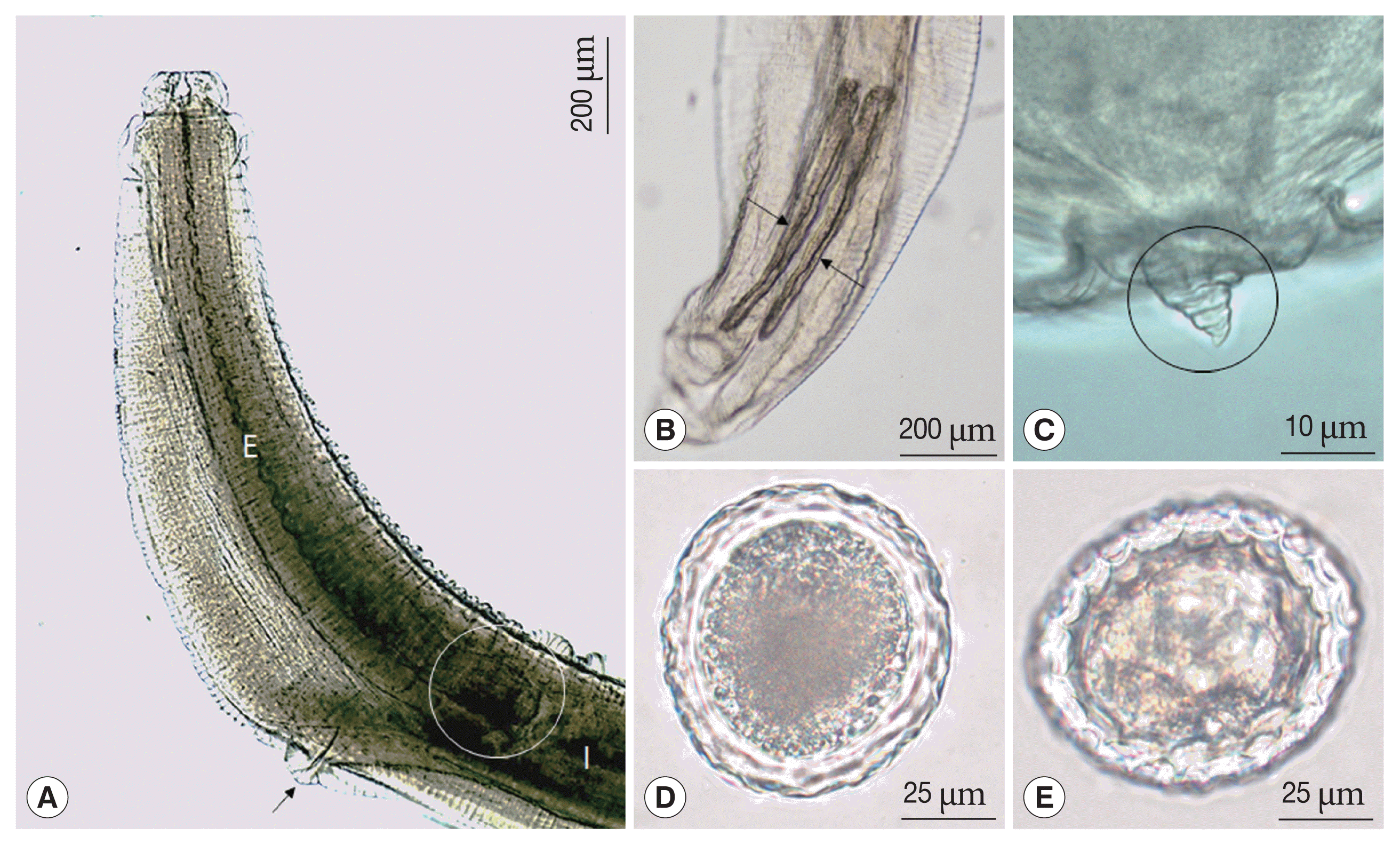
T. apodemi lacks cervical alae. However, our SEM images showed cervical alae as vestigial organs that looked like a slightly uplifted superficial sewing stitch.
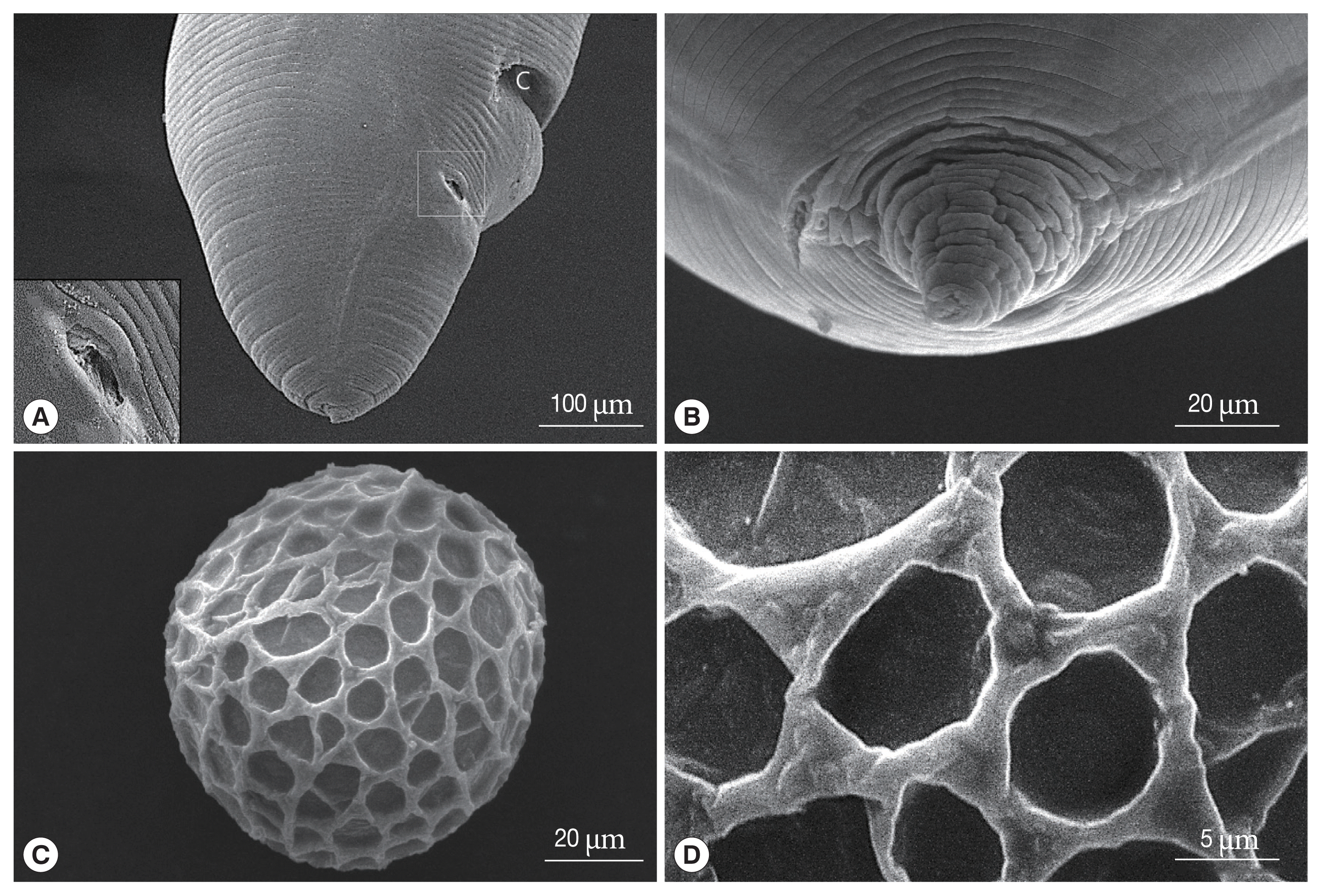
The major and minor axes of
T. canis eggs measure 71.6–91.2 μm by 63.4–79.0 μm,
T. cati eggs measure 63.7–88.1 μm by 53.3–73.3 μm, and
T. malaysiensis eggs measure 60–68 μm by 68–76 μm [
3,
19]. In our study, the eggs measured 73–82 μm. Although the eggs observed in this study were smaller than the
T. canis eggs and bigger than
T. malaysiensis eggs, other significant differences were not observed. SEM images showed that the
Toxocara eggs with an elevated albuminous coat were similar in shape to those of other members of the genus
Toxocara [
19]. Further, the surface of the egg observed in our study was coarsely pitted and covered with elevated albumin, similar to that of other toxocarids [
1].
The tail of the male has a curved posterior end that distinguishes it from the straight-tailed female. The posterior end of the female is terminating in a conical shape with a retractile spine-like structure in
T. apodemi and
T. indica, but that of other species (i.e.,
T. malaysiensis,
T. lyncis,
T. tanuki) are conical [
1,
3,
8,
11,
12]. Similar to
T. indica, the sampled individuals had 6 pairs of postcloacal papillae, as opposed to the 5 pairs observed in
T. tanuki and
T. vitulorum [
11,
12]. Further, we observed that the posterior end of the female had a conical, retractile spine-like structure, which is a characteristic feature of
T. apodemi [
1].
Fagerholm [
20] reported that the precloacal median papilla in the males of genus
Toxocara was simple and surrounded by a cuticle of bifid appearance. Sanmartín et al. [
21] also indicated that the precloacal median papilla was highly variable in the genus
Toxocara, but the cuticle of bifid appearance in
T. genettae was not observed. In
T. apodemi, Olsen [
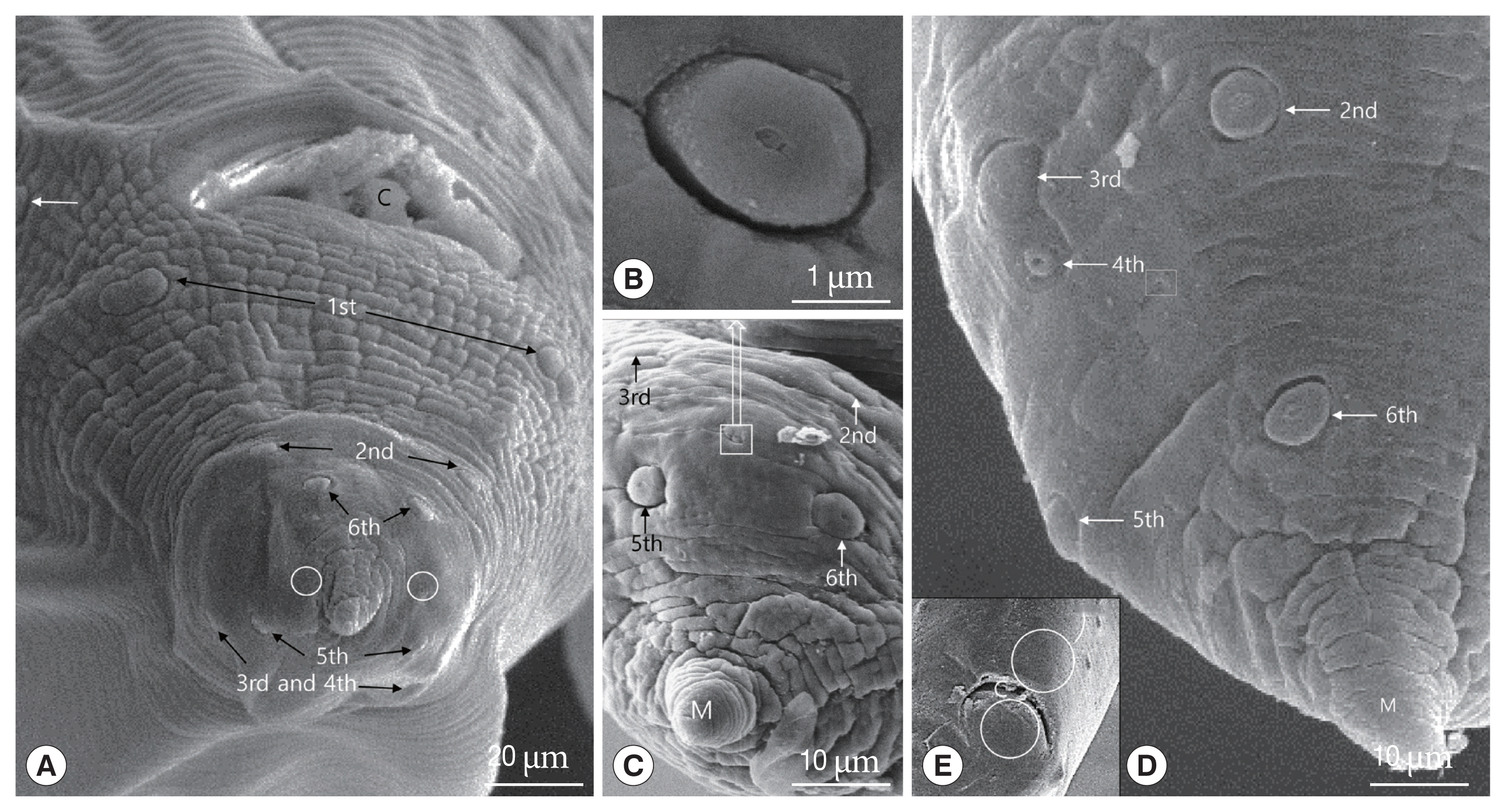
13] described that 12 to 14 pairs of pre-anal (precloacal) papillae and 6 pairs of post-anal (postcloacal) papillae are present, the posterior 3 pairs of the post-anal papillae being located on the lob-like terminal portion of the tail. The 3th, 4th, and 6th pairs of these papillae are laterally located, while the 2nd and 5th pairs are sub-ventral, the 2nd pair consisting of double papillae. The 1st pair is also on the ventral surface, but much closer to the lateral margin [
13]. In this study, the 1st pair of postcloacal papilla numbered by Olsen [
13] was located on the ventrolateral portion in front of cloaca, i.e., the papilla was precloacal papilla. Therefore, we numbered the 1st pair of postcloacal papilla from the double papillae, which was numbered the 2nd pair by Olsen [
13], as the 1st pair of postcloacal papilla. Especially, the 3rd and 4th postcloacal papillae were closely associated with each other, and the 4th pair of post cloacal papillae were smaller than other papillae. The 4th pair of this study was not described by Olsen [
13].
The precloacal median papilla and a single postcloacal median papilla were found in
T. tanuki [
11]. However, like
T. indica [
12], we did not observe the precloacal and postcloacal median papillae in the present study.
Sanmartín et al. [
21] discussed that the eggs of
T. genettae are similar in shape and surface structure to those of other members of the genus, and their size (64–74×50–54 μm) is similar to that of
T. cati, and measurements of the superficial pits of egg (4.8×3.2 μm) are similar to those of
T. canis, but slightly smaller. In this study, the size of egg (73–82 μm) and the superficial pits of egg (mean 8.6×6.7 μm) are obviously bigger than those of
T. genettae,
T. cati and
T. canis.
For the identification of parasites, the 18S rRNA gene was analyzed as it is highly conserved and contains variable regions flanked by more conserved regions [
18,
22]. Although partial sequences of the 18S rRNA gene of
T. apodemi were similar to those of genus
Toxocara, they were not perfectly matched with other members of
Toxocara and a few unique variable regions were observed. Since there are not many sequences in the
Toxocara clade, the morphology and gene of
T. apodemi strongly support that this parasite is close to the
Toxocara clade.
In conclusion, this is the first study to characterize T. apodemi using morphological features, SEM, and sequencing analysis. In SEM images, the cervical alae of T. apodemi was showed as the vestigial organs that looked like a slightly uplifted superficial sewing stitch. Also, we firstly found out the smaller 4th pair of postcloacal papilla, which is closely associated with 3rd papilla. In particular, the 3rd, 4th, and 5th pairs of postcloacal papilla is situated on the latero-dorsal surface than on the latero-ventral surface. The eggs of T. apodemi are easily distinguished from those of other Toxocara species by the bigger superficial pits. Although the partial 18S rRNA sequence of T. apodemi showed high homology of other Toxocara species, we found that the sequence of T. apodemi is not totally matched with those of other Toxocara species. It was confirmed that ascarid nematodes, T. apodemi, recovered from striped field mice in Korea are taxonomically conspecific relationship with genus Toxocara and genetic divergence from other Toxocara species.