INTRODUCTION
Among the 37-collar-spined
Echinostoma spp. or ‘
Echinostoma revolutum group’, total 26 valid or validity-retained spp. were acknowledged worldwide [
1]. They included 16 valid species, i.e.,
E. revolutum,
E. bolschewense,
E. caproni,
E. cinetorchis,
E. deserticum,
E. lindoense,
E. luisreyi,
E. mekongi,
E. miyagawai,
E. nasincovae,
E. novaezealandense,
E. paraensei,
E. paraulum,
E. robustum,
E. trivolvis, and
Echinostoma sp. IG of Georgieva et al., 2013, and 10 validity-retained species., i.e.,
E. acuticauda,
E. barbosai,
E. chloephagae,
E. echinatum,
E. jurini,
E. nudicaudatum,
E. parvocirrus,
E. pinnicaudatum,
E. ralli, and
E. rodriguesi [
1]. Recently, however,
E. robustum Yamaguti, 1935 has been put to a synonymy with
E. miyagawai Ishii, 1932 [
2]. Eight among the 26 species, including
E. revolutum,
E. cinetorchis,
E. echinatum,
E. lindoense,
E. mekongi,
E. miyagawai (experimental infection),
E. paraulum, and possibly
E. paraensei (from the coprolite of a human mummy), are regarded as human-infecting 37-collar-spined echinostomes [
1].
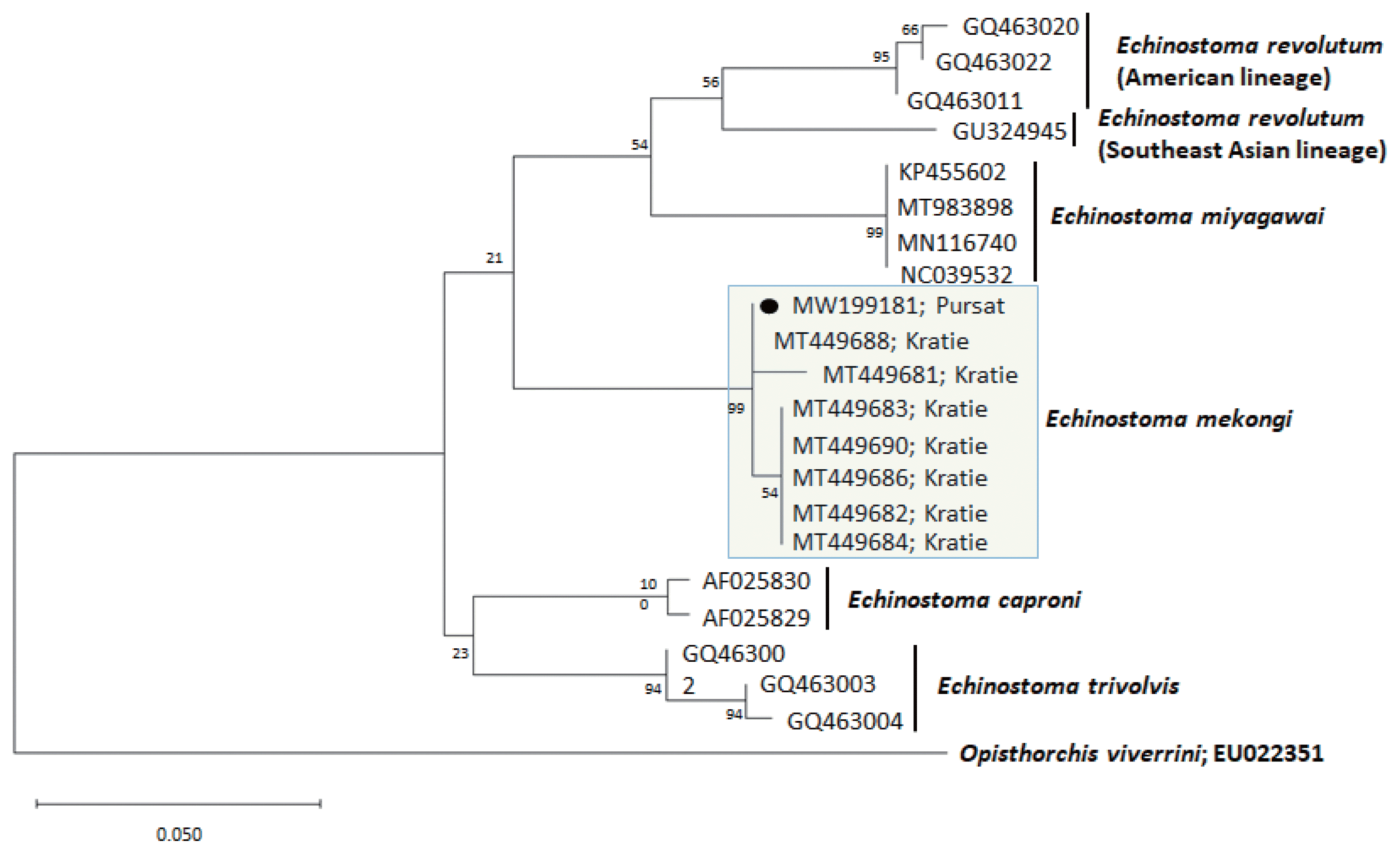
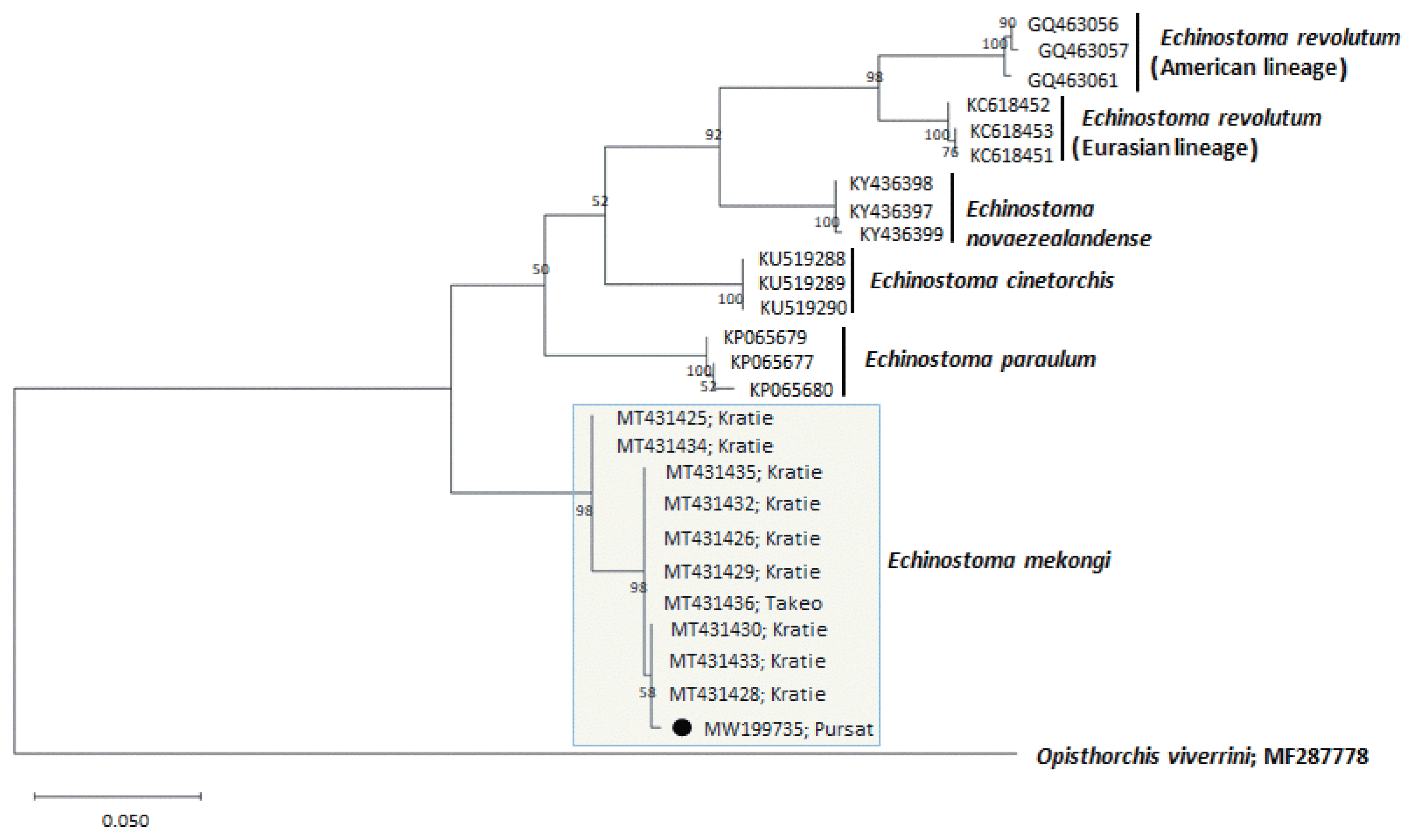
Echinostoma mekongi Cho, Jung, Chang, Sohn, Sinuon& Chai, 2020 (Digenea:
Echinostomatidae) was described as a new species based on adult flukes collected from 6 riparian people in Kratie and Takeo Province, Cambodia [
3]. This species was morphologically characterized by a small head collar, small collar spines, small oral and ventral suckers, and small cirrus sac compared to
E. revolutum or
E. miyagawai, and marked variation was noted in the morphology of testes, which are either globular or lobulated [
3]. However, its life history, including the first and second intermediate hosts and animal reservoir hosts as well as epidemiological characteristics, including geographical distribution, remained unknown. In the present study, we performed a life cycle study of
E. mekongi and discovered its metacercarial stage in a species (subspecies) of freshwater snail,
Filopaludina martensi cambodjensis, purchased from a local market in Pursat Province, Cambodia and obtained adult flukes through experimental infection to a hamster. The adult flukes were morphologically and molecularly (
cox1 and
nad1) confirmed to be
E. mekongi Cho et al., 2020 [
3].
MATERIALS AND METHODS
Ethical issue
To recover the adult flukes of E. mekongi, animal experiment was performed using a laboratory-bred Syrian golden hamster (Mesocricetus auratus). This animal experiment followed the guidelines of the Committee on the Ethics of Animal Experiments in Gyeongsang National University College of Medicine, Jinju, Korea (2017).
Collection of the metacercariae
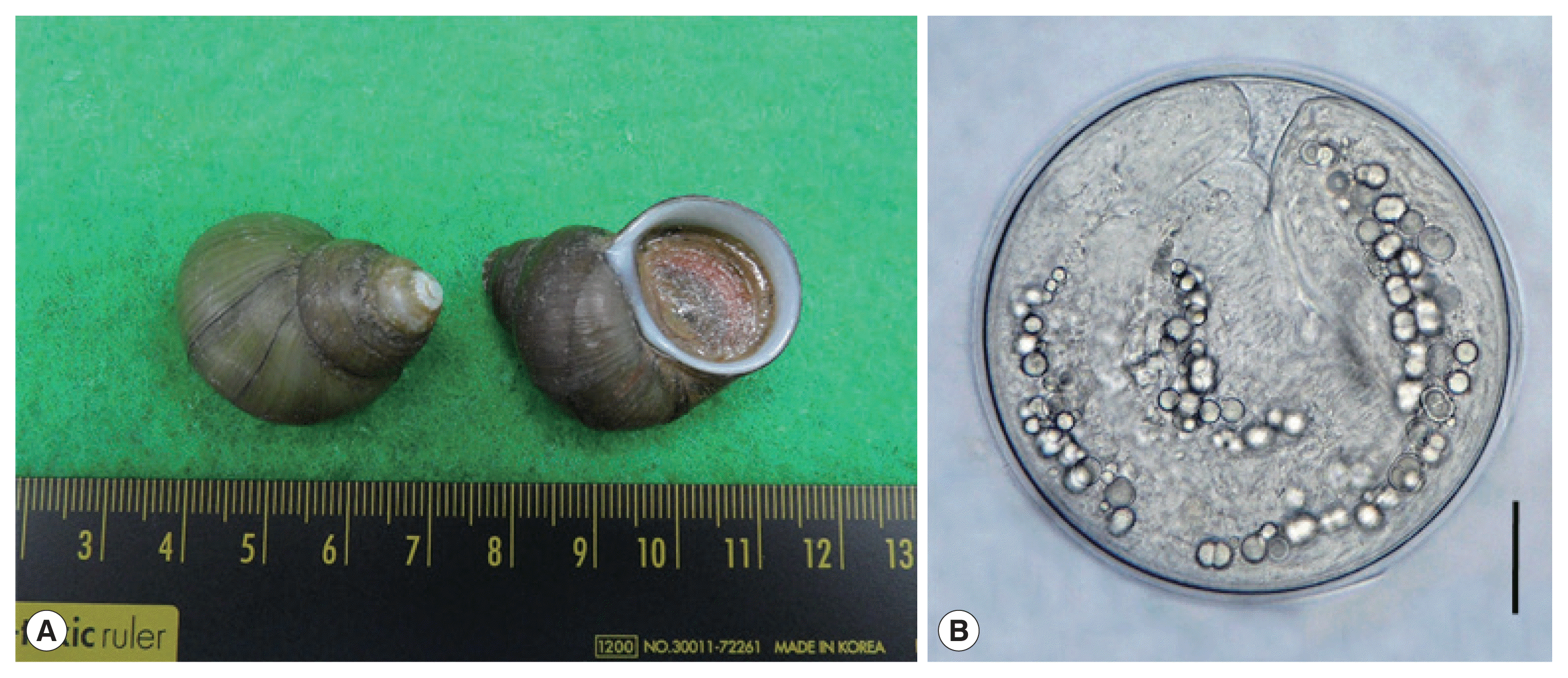
We purchased freshwater snails,
F. martensi cambodjensis (
Fig. 1A), from a local market in Pursat Province, Cambodia in May 2017. The identification of the snail species and subspecies was performed according to a previous report [
4]; in comparison with
Filopaludina martensi martensi, this subspecies is lacking in spiral ridges. The snails were transported to Korea (Department of Parasitology and Tropical Medicine, Gyeongsang National University College of Medicine, Jinju) under ice, and examined for echinostome metacercariae using the artificial digestion method as previously described [
5]. After crushing their shells with a mortar and pestle, they were digested with pepsin-HCl solution for 2 hr. Metacercariae were collected from the digested material under a stereomicroscope (Olympus, Tokyo, Japan).
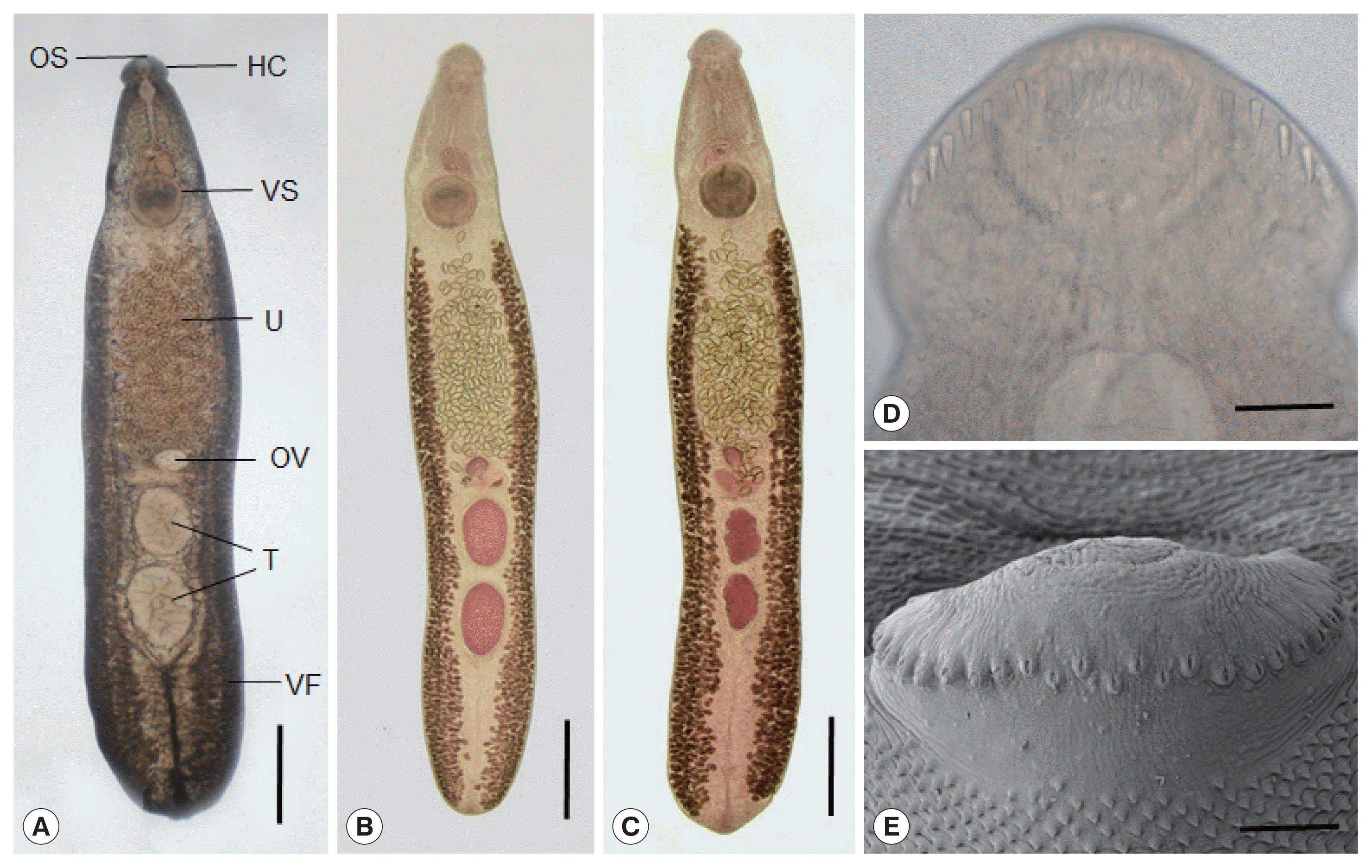
The metacercariae were morphologically observed under a light microscope (Olympus). They (75 in number) were orally infected to an experimental hamster, and adult flukes were recovered in the small intestine at day 20 after the infection. The adult flukes were fixed with 10% neutral buffered formalin under a cover slip pressure, stained with Semichon’s acetocarmine, cleared in glycerin-alcohol, and mounted in glycerin-jelly for light microscopic observations. Some adult flukes were fixed in 70–80% ethanol for molecular analyses. To observe the surface ultrastructure, some adult flukes were washed several times with 0.2 M cacodylate buffer (pH 7.2), fixed with 2.5% glutaraldehyde at 4˚C, and washed 3 times with the buffer. They were then dehydrated through a graded ethanol series (50%, 70%, 80%, 90%, 95%, and absolute alcohol), dried with hexamethyldisilazane, coated (JFC-1100E ion sputtering device, Jeol, Tokyo, Japan) with gold, and observed using a scanning electron microscope (Jeol JSM-7610F, Tokyo, Japan) at an accelerating voltage of 15 kV.
Molecular analyses (cox1 and nad1)
For molecular analyses, worms preserved in ethanol were used. Genomic DNA was extracted using the Spin-Column Protocol of DNeasy® Blood&Tissue kit (Qiagen, Hilden, Germany). PCR was then conducted using specific primers designed to amplify
cox1 and
nad1 genes in echinostomes. The primers for
cox1 gene were JB3 and JB13, and the primers for
nad1 were JB11 and JB12 [
6]. The PCR products were purified and directly sequenced by Macrogen Inc. (Seoul, Korea). For evaluation of the genetic identity, the basic local alignment search tool (BLAST;
http://blast.ncbi.nlm.nih.gov/Blast.cgi) was applied. We used the Geneious® version 10.2.6 program (Biometers Ltd., Auckland, New Zealand) for alignment of the obtained sequences with GenBank reference
cox1 and
nad1 sequences of 37-collar-spined
Echinostoma spp. Multiple sequence alignment was performed using Clustal W program [
7]. The phylogenetic tree was constructed using the maximum-likelihood method based on Tamura-Nei model of nucleotide substitution with 1,000 bootstrap replications with our samples and several other 37-collar-spined
Echinostoma spp. deposited in GenBank, and viewed by MEGA X program [
8].
DISCUSSION
The present study first confirmed that
F. martensi cambodjensis, a freshwater gastropod species, acts as a second intermediate host for
E. mekongi in Pursat Province, Cambodia. This snail species (
F. martensi) is widely distributed in Indochina Peninsula, including Vietnam, Thailand, and Cambodia [
4,
9–
11]. In Pursat Province, Cambodia, nearby the Tonle Sap Lake, these snails are commercially available in street markets together with larger snails, including
Pila spp. Local people favor to eat them undercooked. It is thus presumed that
F. martensi cambodjensis snails are a potential source of human infection with
E. mekongi. However, in Kratie and Takeo Provinces where human cases with
E. mekongi were detected [
3], the significance of these snails has not yet been verified, and it is urgently needed to examine these snails for
E. mekongi metacercariae.
The metacercariae of
E. mekongi were almost round (163–190 μm in diameter), equipped with 37 collar spines on the head collar, and morphologically indistinguishable from those of the other 37-collar-spined
Echinostoma spp. However, they were slightly or markedly larger than the metacercariae of
E. lindoense (120–130 μm),
E. revolutum (132–152 μm),
E. miyagawai (144–154 μm), and
E. trivolvis (135–170 μm) [
1].
It is referable that the same or closely related snail species, for example,
F. martensi martensi,
F. sumatrensis polygramma,
F. doliaris, and
Filopaludina sp., act as the second intermediate hosts of the other echinostome species, including
E. revolutum [
9–
11] and
Artyfechinostomum malayanum (syn.
Echinostoma malayanum) [
11]. It is also interesting to note that
F. martensi martensi snails can be used as a biological control agent of
Bithynia siamensis goniomphalos, the first intermediate host of
Opisthorchis viverrini [
12]. This may imply that these 2 trematode species are antagonistic and useful to understand epidemiology and geographical distribution of each species.
As
E. mekongi was originally reported from human infections [
3], and a hamster was susceptible for this echinostome infection in this study, a speculation could be made that
E. mekongi is an echinostome of mammalian hosts. However, many of the other 37-collar-spined
Echinostoma spp. are known to infect both birds and mammals, for example,
E. revolutum,
E. miyagawai, and
E. trivolvis [
1]. Although natural reservoir hosts for
E. mekongi have never been discovered, this may also be applied to
E. mekongi for taking both type of hosts. This point should be clarified by field surveys and also experimentally in the near future.
The first intermediate host of
E. mekongi is unknown. In
E. revolutum,
Lymnaea spp. and
Radix spp. are the most important snail species [
13], whereas, in
E. miyagawai,
Planorbis planorbis,
Anisus vortex, and
Radix peregra snails were reported to be natural first intermediate hosts [
14,
15]. Their cercariae are echinostome-type with an oral sucker armed with a spiny collar, ventral sucker, excretory bladder, and a moderately long tail [
13].
Regarding the morphology of
E. mekongi adults, several distinct characters described in the original report [
3] were reconfirmed in this study, although the size of the adult flukes were larger in specimens from human infections (av. 11.3 mm in length) than those from an experimental hamster in this study (av. 7.3 mm in length, 20-day-old). They morphologically resembled
E. revolutum,
E. miyagawai (syn.
E. robustum),
E. lindoense, and
E. trivolvis. They revealed slight body constriction near the level of the ventral sucker as reported in
E. miyagawai and
E. lindoense but not in
E. revolutum and
E. trivolvis [
1]. The adults of
E. mekongi had a smaller head collar, smaller collar spines, smaller suckers, and smaller cirrus sac compared to
E. revolutum and
E. miyagawai. They (
E. mekongi) also revealed variation in the morphology of testes; globular or slightly to markedly lobulated. Similar testis morphology was reported in
E. revolutum; their 2 testes are either entire or lobated [
16]. However, the testes morphology of
E. mekongi was comparable with that of
E. miyagawai and
E. lindoense; irregularly or deeply lobed (3–5 lobes) at times with horizontal extension in the former [
2] and deeply indented in the latter [
17]. Another unique feature of
E. mekongi adults was the shape of collar spines; they were not so long and not so sharply pointed as those of
E. revolutum,
E. miyagawai, and
E. trivolvis [
3]. In addition, the confluence of 2 lateral groups of vitelline follicles beyond the posterior testis field, which can be seen in
E. lindoense,
E. miyagawai, and
E. trivolvis [
1] was not recognized in
E. mekongi.
It should be referred that, in Pursat Province, Cambodia, human infections with
E. revolutum were previously reported [
18]. The cases were 4 schoolchildren who occasionally experienced vague abdominal pain and discomfort; they were fond of eating undercooked snails or clams of unidentified species caught near the Tonle Sap Lake and sold on the road to their homes after school [
18]. The worms recovered from the children were diagnosed as
E. revolutum based on morphological features [
18]. However, it is now strongly suggested that they might have been
E. mekongi. In particular, the figure in the paper shows a body constriction near the ventral sucker level in adult worms and relatively short and not sharply pointed collar spines on the head collar [
18] which is consistent with
E. mekongi rather than
E. revolutum. However, it is regretted that molecular analyses on the worms were not performed [
18]. Studies on human infections with
E. mekongi should be performed in Pursat Province and other localities of Cambodia as well as in different countries of Indochina Peninsula.