Introduction
Many people live together with companion animals at home. Among the different species of household pets, dogs are the most popular, and their population is increasing annually. In contrast, the number of abandoned dogs is also increasing, with most being stray dogs. Many abandoned dogs and/or stray dogs are detained in animal shelters in Korea. Notably, dogs act as the reservoir hosts of zoonotic intestinal parasites in individual houses and in shelters. Intimate contact with dogs can be an avenue for the transmission of zoonotic diseases, including intestinal parasite infections [1]. Thus, there is an urgent need for comprehensive epidemiological studies that encompass both pet and shelter dogs. These studies should be aligned with the One Health approach, which considers the interconnected health of humans, animals, and the environment. Additionally, it is important to develop a robust surveillance system for pet owners for the prevention and management of zoonotic diseases, thereby safeguarding public health.
Zoonotic intestinal parasitic infections, such as those caused by helminths and protozoa, are typically transmitted through the ingestion of contaminated water or food, causing diseases such as traveler’s diarrhea in humans. Previous studies in Korea have identified a range of intestinal parasites in dogs, including various species of helminths [2–5]. Among the different protozoa, Giardia duodenalis is a significant enteric pathogen that affects both companion animals and livestock, with a particularly high prevalence in Korean pet dogs [6,7]. Additionally, Cryptosporidium spp. infections have been well-documented in both domestic animals and humans in Korea [8]. However, there is still a lack of comprehensive national epidemiological surveys on intestinal protozoan infections, highlighting the need for further research in this area.
The primary objective of this study was to investigate the current status of intestinal parasite infections in dogs across Korea through the analysis of fecal samples. Specifically, we identified the prevalence of zoonotic intestinal parasites, in the hopes of using these findings as foundational data for the development of a comprehensive surveillance system for dog-related zoonoses in Korea. This study seeks to contribute toward the prevention and management of zoonotic diseases associated with dogs by determining their distribution and identifying critical zoonotic pathogens.
Materials and Methods
Ethical approval
Ethical approval for all experimental procedures was granted by the Institutional Animal Care and Use Committee at Kyungpook National University (approval no. KNU 2022-0442). No animals were harmed during the collection of fecal samples; specimens were obtained noninvasively without needing any special interventions.
Collection of fecal samples
The sample size for this study was calculated using power analysis based on an anticipated disease prevalence of 20%, a permissible absolute error of 5%, and a confidence level of 95%, employing a simple random sampling approach [9]. A minimum of 246 samples was deemed necessary. Fecal samples were collected from the northern (Seoul and Gyeonggi-do), central (Chungcheong-do), and southern (Gyeongsang-do) regions of the Korean peninsula. Samples were gathered evenly from these regions, as well as from both shelter and pet dogs. From 2022 to 2023, a total of 367 fecal samples from clinically normal dogs (184 stray dogs, 183 pet dogs) were collected and analyzed. This diverse sample pool was structured to facilitate a comprehensive comparative analysis across multiple variables, including age, gender, season, breed, source, and region.
Stool examination using the saturated sodium nitrate flotation technique
Fresh fecal samples were promptly collected and stored at 4°C in sterile tubes to maintain integrity until processing, which was done within 2 days of collection. The presence of intestinal helminths (e.g., roundworms, whipworms, hookworms, and tapeworms) and protozoa (e.g., coccidia and Giardia) was assessed using the saturated sodium nitrate flotation technique [10], which leverages the natural buoyancy of parasitic eggs and oocysts to effectively facilitate their separation and identification. While the sedimentation technique is recognized for its ability to detect certain heavier helminth eggs, the flotation method was used for its established efficacy in routine diagnostic applications and its capacity to produce clear and reliable results in large-scale epidemiological studies.
Molecular genetic examination of Giardia and Cryptosporidium spp
Genomic DNA was extracted from fecal samples using the QIAamp Fast DNA Stool Mini Kit (Qiagen, Melbourne, Australia) according to the manufacturer’s instructions. PCR amplification was done using the AccuPower HotStart PCR Premix Kit (Bioneer, Daejeon, Korea). Nested PCR was used to amplify the 18S rRNA gene for detecting Cryptosporidium spp., producing a fragment approximately 587 bp in length with the primer sets 18SiCF2/18SiCR2 and 18SiCF1/18SiCR1 [11]. For G. duodenalis, DNA samples were subjected to nested PCR using 2 different protocols based on the target gene. A 530-bp fragment of the gdh gene was amplified using external primers (Gdh1 and Gdh2) and internal primers (Gdh3 and Gdh4) [12]. Thereafter, positive samples were retested using a set of primers designed to amplify a 174-bp fragment of the 18S rRNA gene, specifically external primers (RH11 and RH4LM) and internal primers (GiarF and GiarR) [13].
DNA sequencing and phylogenetic analysis
Sequencing of the positive samples was conducted at Macrogen (Seoul, Korea) using the specific primer sets. The subsequent comparative analysis was performed utilizing GenBank nucleotide sequences from the NCBI database. Sequence alignments were performed using CLUSTAL Omega (ver 1.2.1), with further editing using BioEdit (ver 7.2.5). Phylogenetic trees were constructed using MEGA (version 6.0), applying the maximum likelihood method and the Kimura two-parameter distance model. The sequence alignment involved a similarity matrix, with the phylogenetic tree’s stability evaluated using a bootstrap analysis with 1,000 replicates.
Results
Overall prevalence of intestinal parasites
Over a 1-year period, a total of 367 fecal samples were collected and analyzed, yielding an overall positivity rate of 32.3% (118/367) for 8 types of intestinal helminths (Toxocara canis, Toxascaris leonina, Trichuris vulpis, Ancylostoma caninum, and Spirometra spp.) and protozoa (Cystoisospora spp., Cryptosporidium spp., and Giardia spp.). Significant differences in infection rates were noted based on several factors. Regarding breed, mixed-breed shelter dogs had a higher positivity rate at 36.7% (72/196) versus pet dogs (χ2=14.605, df=1, P=0.0001). Regarding age, puppies in shelters exhibited the highest positivity rate at 91.7% (11/12) (χ2=47.675, df=3, P<0.0001), while junior dogs showed a higher positivity rate among pet dogs at 14.7% (5/34) (χ2=15.657, df=3, P=0.0013).
Prevalence of intestinal parasites by stool examination
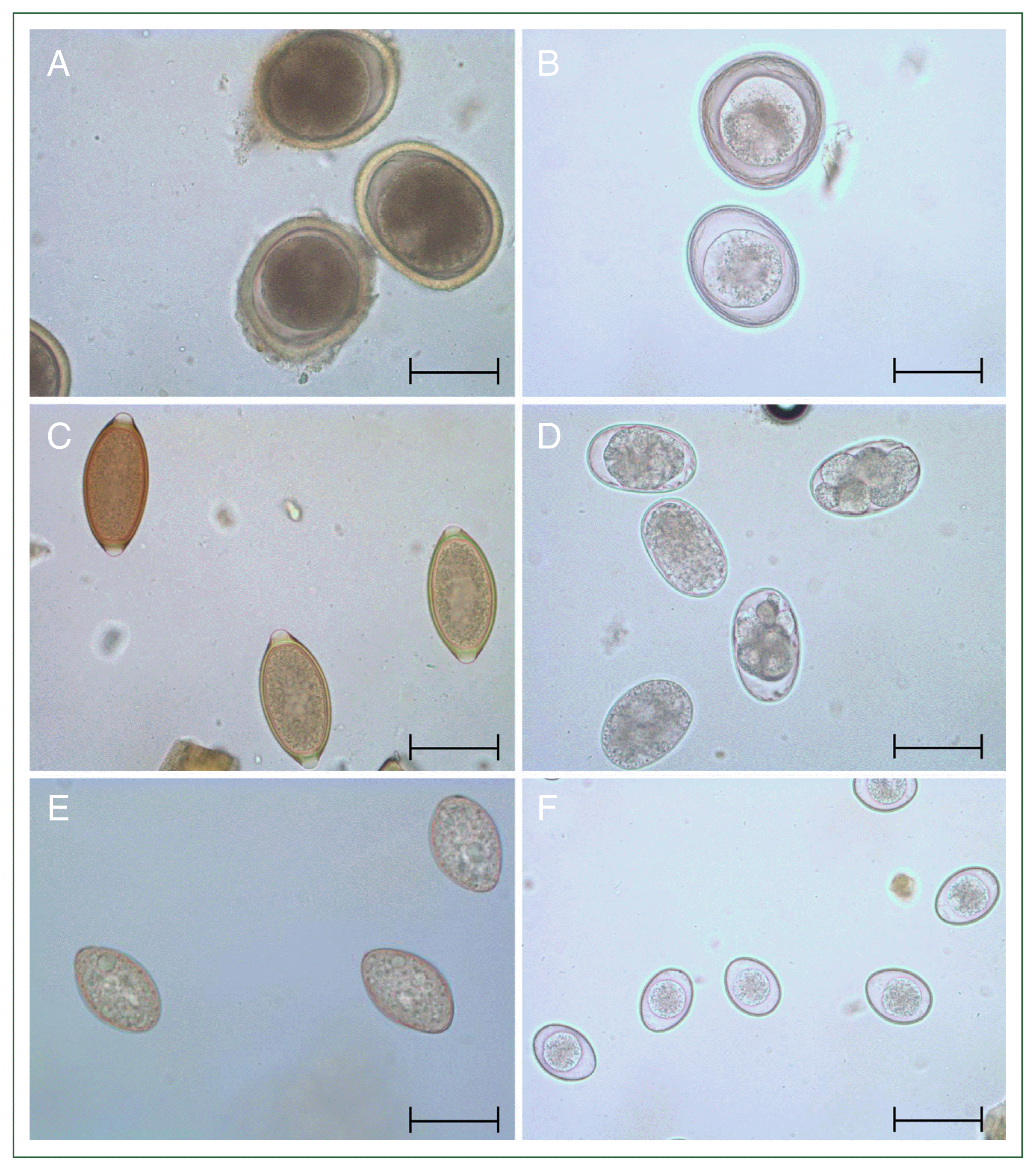
When categorizing the infection rates of 6 specific intestinal parasites, egg examination revealed that Cystoisospora spp., which is not initially a primary focus as a common zoonotic parasite, exhibited the highest infection rate at 7.6% (28/367). As shown in Table 1, this infection rate was higher than that of T. canis (6.0%, 22/367), T. leonina (1.1%, 4/367), T. vulpis (6.8%, 25/367), A. caninum (2.7%, 10/367), and Spirometra spp. (1.1%, 4/367). Neither Giardia nor Cryptosporidium cysts were detected on egg examination. Microscopy helped identify the eggs and oocysts of various species, including T. canis (Fig. 1A), T. leonina (Fig. 1B), T. vulpis (Fig. 1C), A. caninum (Fig. 1D), Spirometra spp. (Fig. 1E), and Cystoisospora spp. (Fig. 1F).
The overall infection rate for roundworms was 7.1% (26/367), specifically 6.0% for T. canis and 1.1% for T. leonina, as detailed in Table 1. For T. canis, in terms of breed, mixed-breed shelter dogs had a significant higher prevalence rate (9.2%, 18/196) than mixed-breed pet dogs (χ2=16.514, df=1, P<0.0001). Regarding age, shelter dog puppies had a significantly higher prevalence rate at 33.3% (4/12) (χ2=31.288, df=3, P<0.0001), while in pet dogs, the junior age group had a significantly higher prevalence rate at 11.8% (4/34) (χ2=39.608, df=3, P<0.0001). Whereas, T. leonina was only detected in shelter dogs.
Among the whipworms, T. vulpis had an infection rate of 6.8%, as detailed in Table 1. Regarding breed, mixed-breed shelter dogs exhibited a significantly higher positivity rate of 9.2% (18/196) versus purebred shelter dogs (χ2=9.344, df=1, P=0.0022).
Among hookworms, A. caninum had an infection rate of 2.7%, as indicated in Table 1. A. caninum was detected exclusively in shelter dogs. Mixed-breed shelter dogs exhibited a significantly higher positivity rate of 4.6% (9/196) versus purebred shelter dogs (χ2=5.532, df=1, P=0.0187).
Among tapeworms, Spirometra spp. had an infection rate of 1.1%, as reported in Table 1. Spirometra spp. were found solely in the shelter dogs. In terms of regional distribution, the rate was significantly higher among shelter dogs in the southern region, at 2.8% (4/143) (χ2=6.335, df=2, P=0.0421).
All coccidia detected on egg examination were identified as Cystoisospora spp., exhibiting a 7.6% infection rate, as shown in Table 1. Regarding breed, purebred shelter dogs displayed a significantly higher positivity rate of 15.2% (26/171) versus mixed-breed shelter dogs (χ2=28.932, df=1, P<0.0001). Among pet dogs, only a single mixed-breed dog tested positive, at a rate of 0.5% (1/196). In terms of age, shelter dogs younger than 3 months showed a significantly higher positivity rate at 25.0% (3/12) (χ2= 12.046, df=3, P=0.0072), while the single pet dog that tested positive belonged to the 3–7-month age range, with a rate of 2.9% (1/34), which was also statistically significant (χ2=9.821, df=3, P=0.0202).
Prevalence of intestinal protozoa on molecular examination
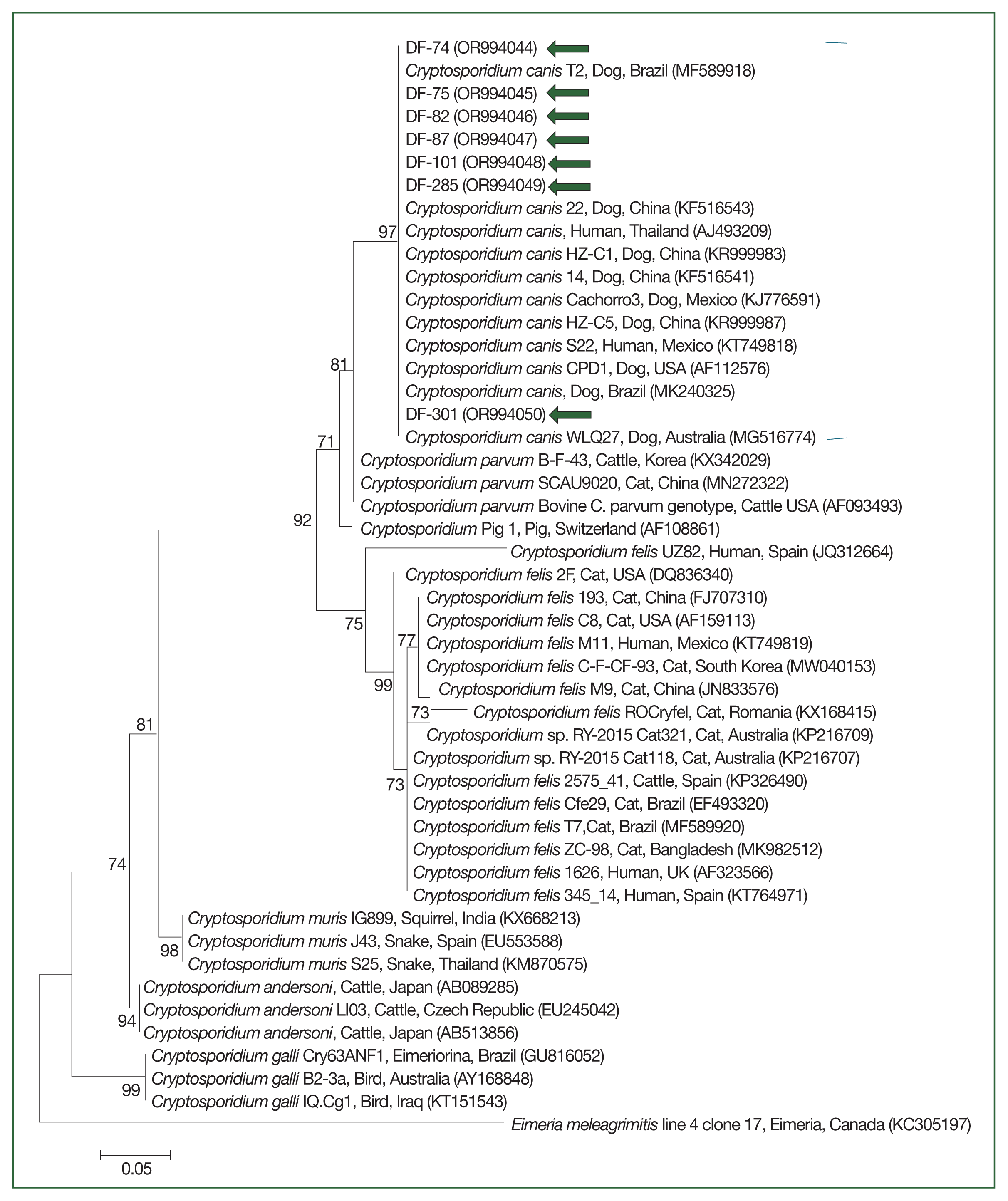
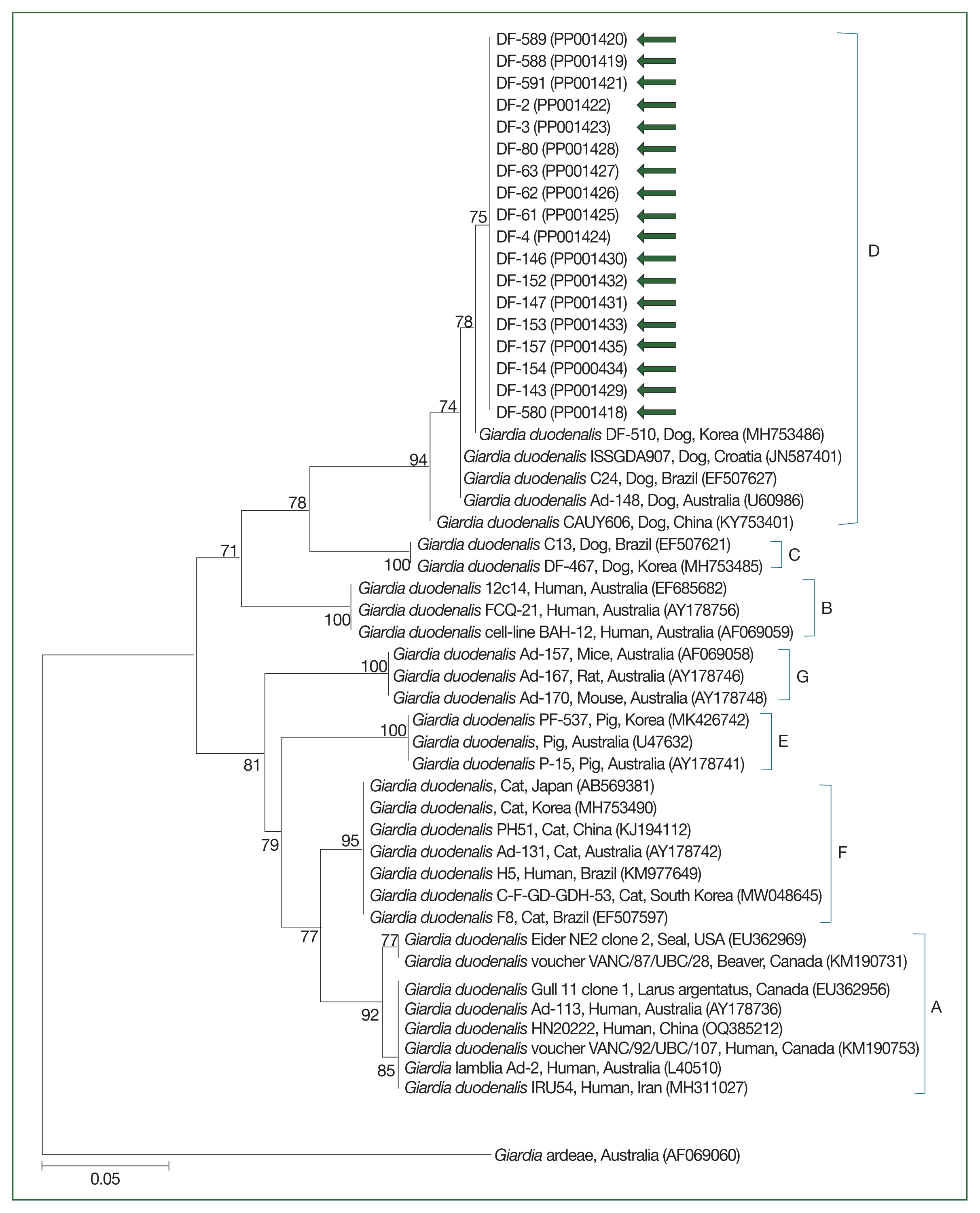
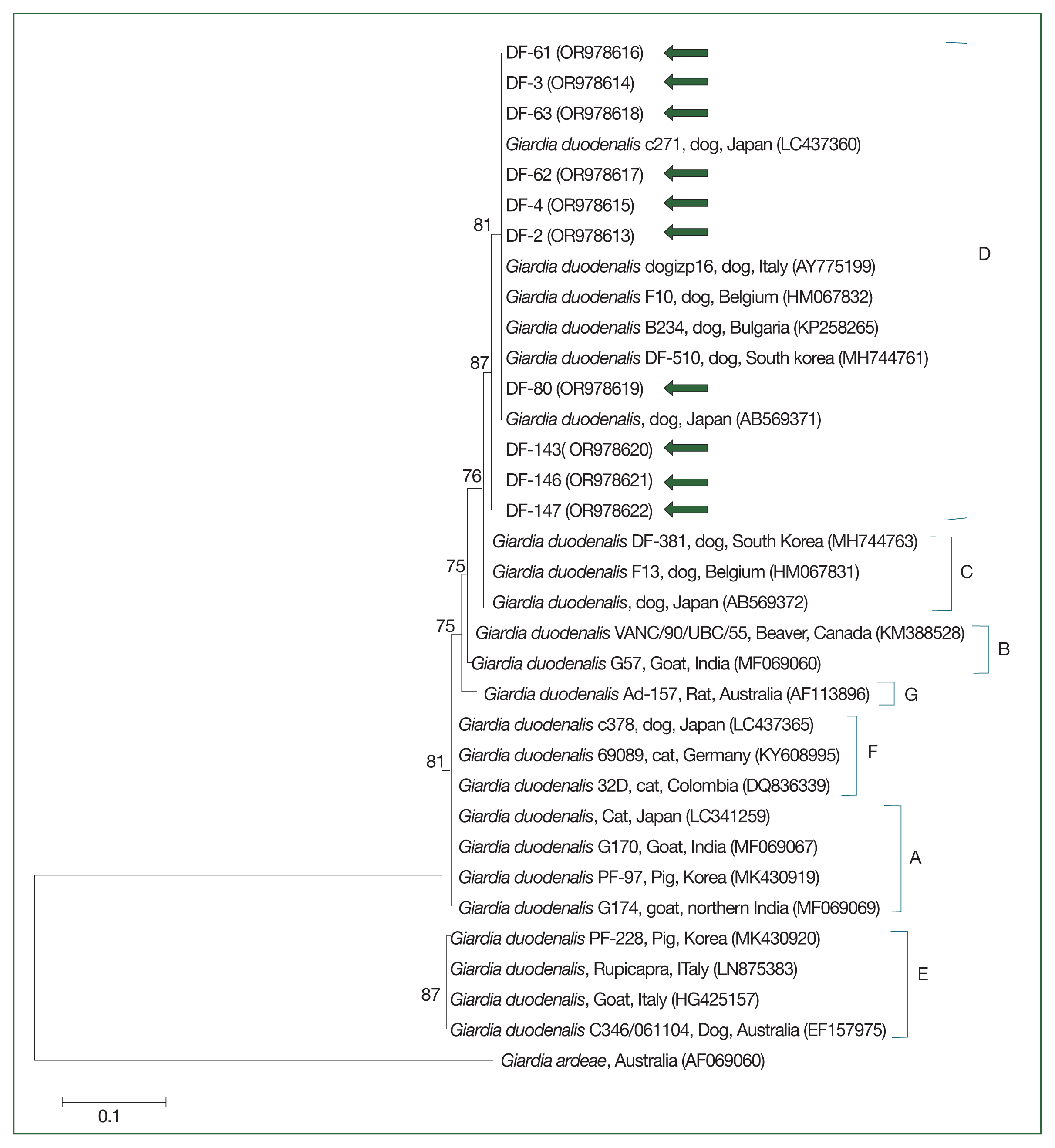
The 18S rRNA gene sequences for Cryptosporidium spp. (Fig. 2) and the gdh (Fig. 3) and 18S rRNA gene sequences for G. duodenalis (Fig. 4) were analyzed. Seven 18S rRNA gene sequences of Cryptosporidium spp. that confirmed the presence of C. canis showed 100% sequence identity among themselves. These sequences also exhibited a similarity of 97.9–99.8% with the 11 previously reported 18S rRNA sequences of C. canis in GenBank (Fig. 2). Similarly, the 18 gdh gene sequences of G. duodenalis analyzed were identical to each other, sharing a similarity of 99.4–99.6% with the 5 previously reported gdh gene sequences of G. duodenalis assemblage D in GenBank (Fig. 3). Additionally, the 11 representative 18S rRNA gene sequences of G. duodenalis identified in this study had a similarity of 99.4–100% with each other and shared a similarity of 99.4–99.6% with the 6 previously reported 18S rRNA sequences of G. duodenalis assemblage D in GenBank (Fig. 4). The sequences identified in this study have been deposited in GenBank under the accession numbers OR994044–OR994050 for the C. canis 18S rRNA gene, PP001418–PP001435 for the G. duodenalis gdh gene, and OR978613–OR978622 for the G. duodenalis 18S rRNA gene.
Species-specific PCR amplifications identified protozoa such as G. duodenalis and Cryptosporidium spp. in canine fecal samples. G. duodenalis had an infection rate of 4.9% (18/367), as shown in Table 2. Mixed-breed shelter dogs showed a significantly higher positivity rate of 6.6% (13/196) versus purebred shelter dogs (χ2=11.758, df=1, P=0.0006). In terms of age, shelter puppies had a significantly higher positivity rate at 25.0% (3/12) (χ2 =17.327, df=3, P=0.0006), whereas pet dogs aged 8 months to 7 years had a 1.6% positivity rate (4/246). Cryptosporidium spp., specifically C. canis, had a prevalence of 1.9% (7/367), as documented in Table 2. C. canis was found exclusively among shelter dogs. No significant differences were reported across the categories analyzed for C. canis.
Discussion
In this study, we investigated the prevalence of intestinal parasites in dogs across various regions in Korea and found that the overall positive rate was 32.2%. Although this rate is lower than in previous studies [2–5], our results underscore the ongoing presence of intestinal parasites in the canine population. These findings are particularly relevant given the increasing number of companion animals and the growing concern over zoonotic infections associated with dogs.
One of the most important findings is the markedly higher prevalence of intestinal parasites among shelter dogs compared to pet dogs. Shelter dogs exhibited significantly higher rates of infection across several parasite species, including T. canis, T. vulpis, and A. caninum. This pattern is consistent with prior research indicating that shelter dogs are more susceptible to parasitic infections due to factors such as overcrowded conditions, poor sanitation, and higher stress levels [14,15]. These environments are conducive to the spread of infections, leading to a higher prevalence of gastrointestinal parasites. Our study highlights the need for improved management practices in shelters, including regular deworming protocols and better hygiene measures to reduce the burden of parasitic infections.
Regarding breed differences, mixed-breed dogs had a significantly higher prevalence of parasites than purebred dogs in both the shelter and pet environments. This is likely because mixed-breed dogs are abandoned at a higher rate than purebred dogs, leading to increased exposure to various pathogens in the external environment. Age-related trends were also noted, with younger dogs, such as puppies and juniors, exhibiting higher infection rates than older dogs. Previous studies that suggest that younger dogs may have weaker immune systems and higher levels of outdoor activity, thereby increasing their exposure to parasites [16,17].
Among roundworms, T. canis, with a prevalence of 6.0%, was more common in shelter dogs than pet dogs. This parasite is of particular concern due to its zoonotic potential. In line with this, a previous report has found higher infection rates in dogs living in more contaminated environments, such as shelters [14]. T. leonina, although less prevalent at 1.1%, was detected exclusively in shelter dogs, reinforcing the notion that these environments pose a significant risk for parasite transmission.
Trichuris vulpis, the primary whipworm infecting dogs, had a prevalence of 6.8%, which is lower than some historical averages but remains noteworthy, especially because of the higher rates observed in shelter dogs [18]. T. vulpis eggs are known for their environmental resilience, which likely contributes to the higher infection rates in adult dogs and in environments where fecal contamination is common. This finding emphasizes the importance of environmental control measures in managing parasitic infections in shelters.
Ancylostoma caninum, a hookworm, had a prevalence of 2.7%, found exclusively in shelter dogs. This finding suggests a decline compared with historical data, which has reported rates as high as 30.4% [2–5,19]. Nevertheless, this parasite remains a concern, particularly because of its zoonotic potential. The study also noted a seasonal increase in A. caninum infections during the summer months, which is consistent with the parasite’s preference for warm, humid conditions [20]. These results underscore the need for seasonally adjusted management practices, particularly in shelter environments where the risk of transmission is higher.
Interestingly, D. caninum, a common tapeworm in dogs, was not detected in this study. This result contrasts with previous studies and may reflect regional or temporal variations in parasite prevalence. Further research is needed to clarify the factors contributing to this discrepancy. Additionally, Spirometra spp. had a rate of 1.1%, consistent with the lower end of reported prevalence rate [2,3,5,19]. Spirometra spp. infection was only detected in shelter dogs, with 3/4 coming from the same area, while the remaining one was rescued from a different area. All 4 fecal samples were tested immediately upon arrival at the shelter, indicating that they were already infected before coming to the shelter. This is presumed to be because, when roaming outside, shelter dogs are more likely to be exposed to primary sources of infection, such as frogs and snakes.
Cystoisospora spp., identified incidentally during egg examination, had a prevalence of 7.6%. This parasite, although not zoonotic, is commonly found in shelter dogs and contributes to the overall burden of parasitic infections in these environments [3,5,21]. The detection of Cystoisospora spp. underscores the need for comprehensive parasite control programs in shelters, even for non-zoonotic species, to improve overall animal health and welfare.
Our molecular analysis provided significant insights into the prevalence of protozoan parasites, specifically C. canis and G. duodenalis. C. canis was identified at a prevalence of 1.9%, and this marks the first molecular confirmation of this genotype among Korean dogs. C. canis is particularly concerning due to its potential zoonotic impact, especially in immunocompromised individuals. Previous studies have reported Cryptosporidium spp. infection rates in dogs of up to 9.7%, with C. canis being the most detected species [22–25]. Notably, C. canis was only found in shelter dogs, and thus maintaining hygiene in shelters is essential to prevent zoonotic transmission. G. duodenalis had a prevalence of 4.9%, with all cases corresponding to assemblage D, a dog-specific genotype. This finding is lower than the prevalence rates reported in previous studies, which have varied widely depending on the detection methods used [7,26,27]. Nevertheless, the zoonotic potential of G. duodenalis remains a significant public health concern, especially because approximately 50% of infected animals carry zoonotic genotypes [28,29]. This emphasizes the importance of routine screening and preventive measures in both pet and shelter environments to reduce the risk of transmission.
These findings provide a comprehensive overview of the current status of intestinal parasite infections in dogs across Korea. Although the overall prevalence of parasitic infections has decreased compared to historical data, the burden of infection in shelter dogs remains significant, thereby highlighting the ongoing need for targeted control measures. Regular administration of anthelmintics, improved hygiene practices, and seasonally adjusted management strategies should be adopted to mitigate the risk of parasite transmission. Additionally, the identification of zoonotic parasites underscores the importance of ongoing surveillance and public health education to reduce the risk of zoonotic transmission. Future research should focus on expanding the sample size, incorporating more sensitive diagnostic techniques, as well as exploring regional and temporal variations in parasite prevalence. This data can help further refine control strategies and improve canine and public health outcomes.