1. Esnafoglu E, Demir EY, Cetinkol Y, Calgin MK, Erdil A, et al. The seroprevalence of antibodies to
Toxoplasma gondii among children with autism.
Dusunen Adam J Psychiatry Neurol Sci 2017;30(4):309-315
https://doi.org/10.5350/DAJPN2017300404

3. Maenner MJ, Warren Z, Williams AR, Amoakohene E, Bakian AV, et al. Prevalence and characteristics of autism spectrum disorder among children aged 8 years—Autism and Developmental Disabilities Monitoring Network, 11 sites, United States, 2020.
MMWR Surveill Summ 2023;72(2):1-14
https://doi.org/10.15585/mmwr.ss7202a1

5. Nayeri Chegeni T, Sharif M, Sarvi S, Moosazadeh M, Montazeri M, et al. Is there any association between
Toxoplasma gondii infection and depression? A systematic review and meta-analysis.
PLoS One 2019;14(6):e0218524
https://doi.org/10.1371/journal.pone.0218524



7. Galeh TM, Sarvi S, Hosseini SA, Daryani A. Genetic diversity of
Toxoplasma gondii isolates from rodents in the world: a systematic review.
Transbound Emerg Dis 2022;69(3):943-957
https://doi.org/10.1111/tbed.14096


8. Prandota J. Autism spectrum disorders may be due to cerebral toxoplasmosis associated with chronic neuroinflammation causing persistent hypercytokinemia that resulted in an increased lipid peroxidation, oxidative stress, and depressed metabolism of endogenous and exogenous substances.
Res Autism Spectr Disord 2010;4(2):119-155
https://doi.org/10.1016/j.rasd.2009.09.011

12. Syn G, Anderson D, Blackwell JM, Jamieson SE. Epigenetic dysregulation of host gene expression in
Toxoplasma infection with specific reference to dopamine and amyloid pathways.
Infect Genet Evol 2018;65:159-162
https://doi.org/10.1016/j.meegid.2018.07.034


13. Wang T, Sun X, Qin W, Zhang X, Wu L, et al. From inflammatory reactions to neurotransmitter changes: implications for understanding the neurobehavioral changes in mice chronically infected with
Toxoplasma gondii.
Behav Brain Res 2019;359:737-748
https://doi.org/10.1016/j.bbr.2018.09.011


14. Omar SSC, Moklas MAM, Mohtarrudin N, Osman M. Toxoplasma gondii stimulates the behavioural changes of rodents: updated evidence. J Med Biomed Appl Sci 2018;6(9):148-153.
19. Al Malki JS, Hussien NA, Al Malki F. Maternal toxoplasmosis and the risk of childhood autism: serological and molecular small-scale studies.
BMC Pediatr 2021;21(1):133
https://doi.org/10.1186/s12887-021-02604-4



20. Al-Hussainy NH, Al-Saedi AM, Al-Lehaibi JH, Al-Lehaibi YA, Al-Sehli YM, et al. Serological evidences link toxoplasmosis with schizophrenia and major depression disorder.
J Microsc Ultrastruct 2015;3(3):148-153
https://doi.org/10.1016/j.jmau.2015.03.006



21. Alvarado-Esquivel C, Urbina-Álvarez JD, Estrada-Martínez S, Torres-Castorena A, Molotla-de-León G, et al.
Toxoplasma gondii infection and schizophrenia: a case control study in a low
Toxoplasma seroprevalence Mexican population.
Parasitol Int 2011;60(2):151-155
https://doi.org/10.1016/j.parint.2010.12.003


22. Okusaga O, Langenberg P, Sleemi A, Vaswani D, Giegling I, et al.
Toxoplasma gondii antibody titers and history of suicide attempts in patients with schizophrenia.
Schizophr Res 2011;133(1–3):150-155
https://doi.org/10.1016/j.schres.2011.08.006


23. Miman O, Mutlu EA, Ozcan O, Atambay M, Karlidag R, et al. Is there any role of
Toxoplasma gondii in the etiology of obsessive-compulsive disorder?
Psychiatry Res 2010;177(1–2):263-265
https://doi.org/10.1016/j.psychres.2009.12.013


24. Tedla Y, Shibre T, Ali O, Tadele G, Woldeamanuel Y, et al. Serum antibodies to
Toxoplasma gondii and Herpesvidae family viruses in individuals with schizophrenia and bipolar disorder: a case-control study.
Ethiop Med J 2011;49(3):211-220.

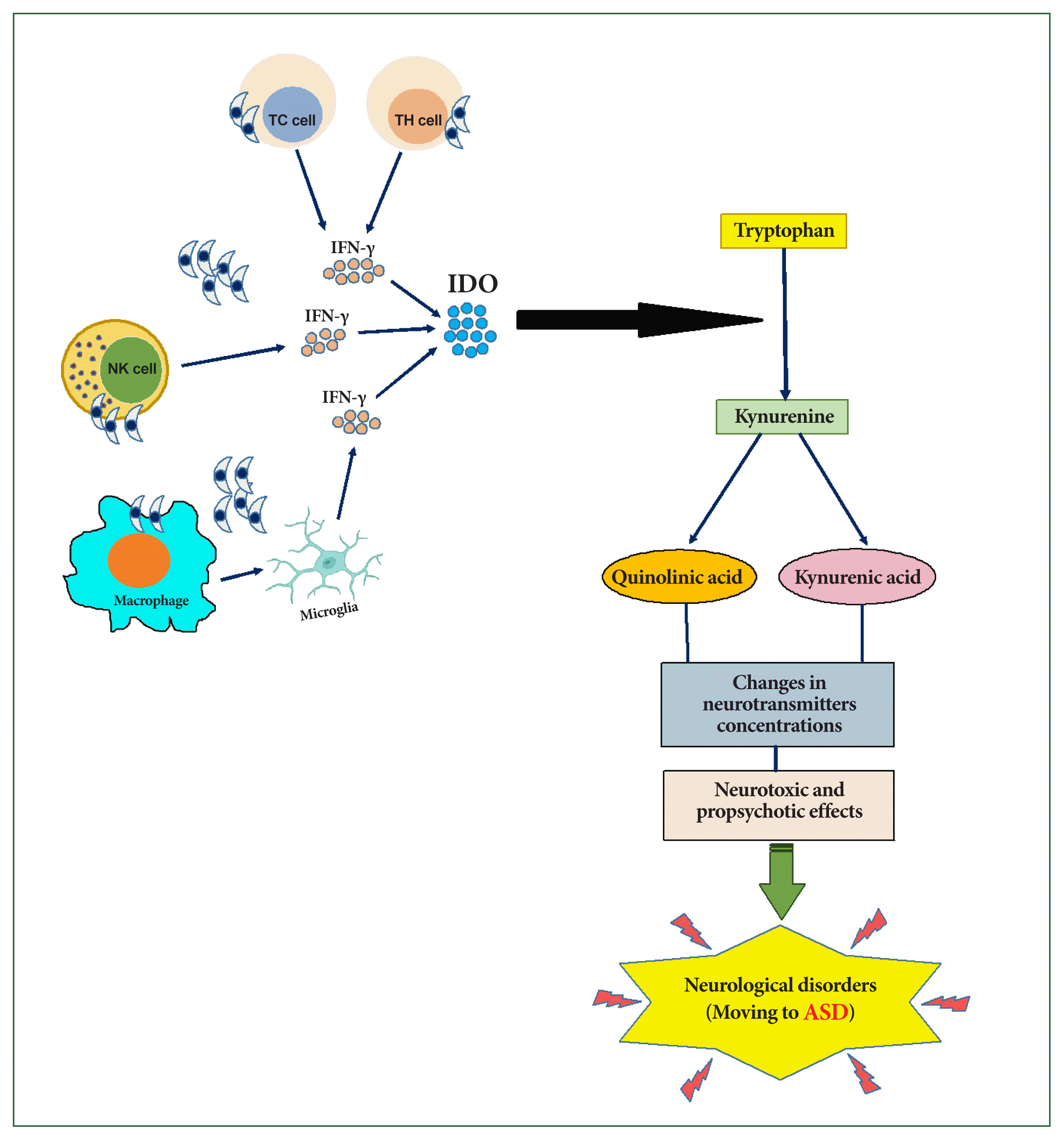
25. Prandota J, Elleboudy NAF, Ismail KA, Zaki OK, Shehata HH. Increased seroprevalence of chronic toxoplasmosis in autistic children: special reference to the pathophysiology of IFN-γ and NO overproduction.
Int J Neurol Res 2015;1(3):102-122.

26. Ramezani M, Shojaii M, Asadollahi M, Karimialavijeh E, Gharagozli K. Seroprevalence of
Toxoplasma gondii in Iranian patients with idiopathic Parkinson’s disease.
Clin Exp Neuroimmunol 2016;7(4):361-365
https://doi.org/10.1111/cen3.12329

28. Pfefferkorn ER. Interferon gamma blocks the growth of
Toxoplasma gondii in human fibroblasts by inducing the host cells to degrade tryptophan.
Proc Natl Acad Sci U S A 1984;81(3):908-912
https://doi.org/10.1073/pnas.81.3.908



30. Engin AB, Dogruman-Al F, Ercin U, Celebi B, Babur C, et al. Oxidative stress and tryptophan degradation pattern of acute
Toxoplasma gondii infection in mice.
Parasitol Res 2012;111(4):1725-1730
https://doi.org/10.1007/s00436-012-3015-6


31. Fujigaki S, Saito K, Takemura M, Maekawa N, Yamada Y, et al. L-tryptophan-L-kynurenine pathway metabolism accelerated by
Toxoplasma gondii infection is abolished in gamma interferon-gene-deficient mice: cross-regulation between inducible nitric oxide synthase and indoleamine-2, 3-dioxygenase.
Infect Immun 2002;70(2):779-786
https://doi.org/10.1128/iai.70.2.779-786.2002



32. Silva NM, Rodrigues CV, Santoro MM, Reis LF, Alvarez-Leite JI, et al. Expression of indoleamine 2, 3-dioxygenase, tryptophan degradation, and kynurenine formation during in vivo infection with
Toxoplasma gondii: induction by endogenous gamma interferon and requirement of interferon regulatory factor 1.
Infect Immun 2002;70(2):859-868
https://doi.org/10.1128/IAI.70.2.859-868.2002



33. Brooks JM, Carrillo GL, Su J, Lindsay DS, Fox MA, et al.
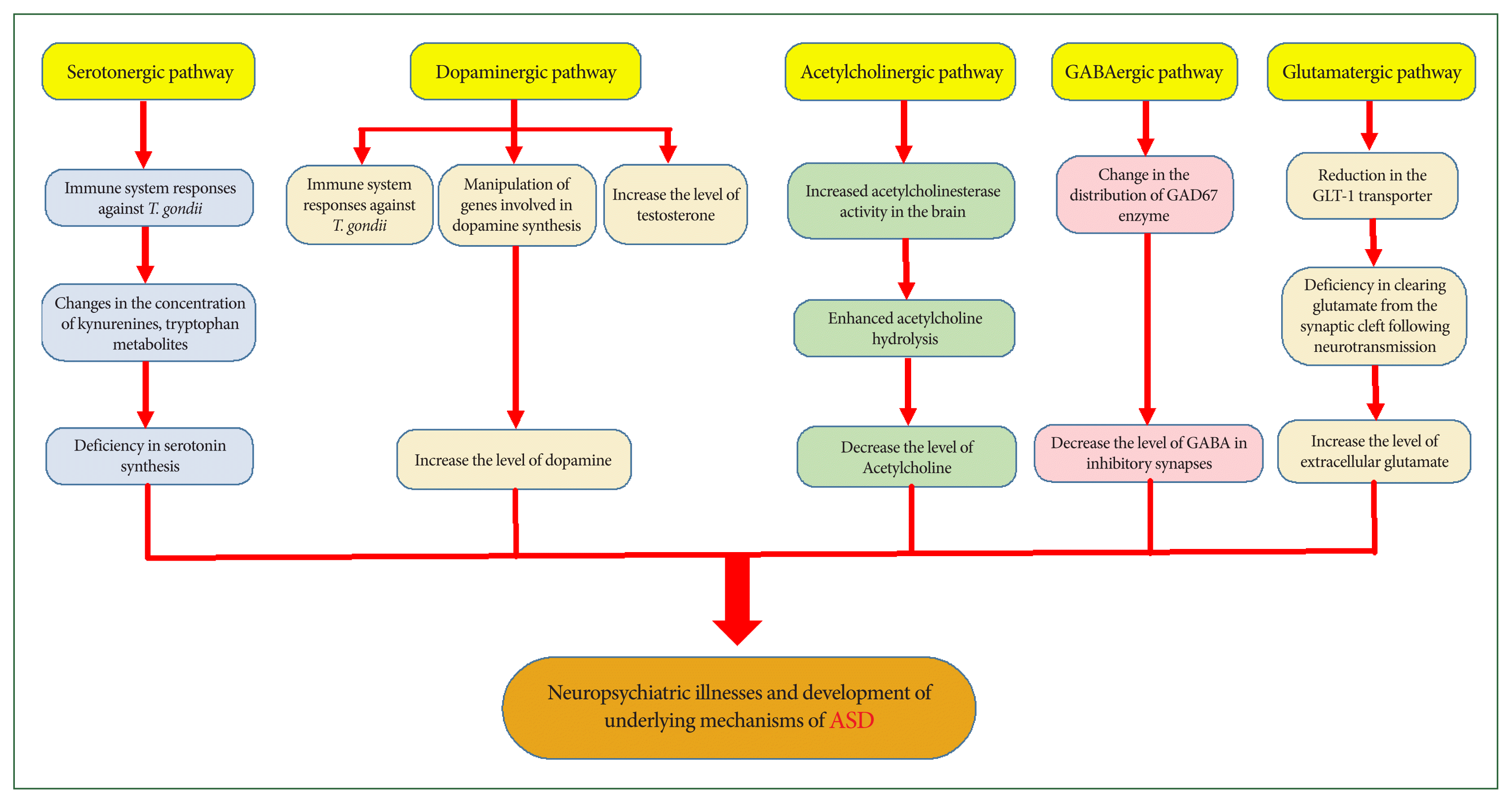
Toxoplasma gondii infections alter GABAergic synapses and signaling in the central nervous system.
mBio 2015;6(6):e01428-15
https://doi.org/10.1128/mBio.01428-15



34. Tonin AA, Da Silva AS, Thomé GR, Sangoi MB, Oliveira LS, et al. Influence of toxoplasmosis on acetylcholinesterase activity, nitric oxide levels and cellular lesion on the brain of mice.
Pathol Res Pract 2014;210(8):526-532
https://doi.org/10.1016/j.prp.2014.04.025


35. AL-Hadad MTS, Kadhim RA, Al-Rubaye AF. Effect of chronic toxoplasmosis on levels of some neurotransmitters (dopamine, adrenaline, and noradrenaline) in human serum. J Pharm Sci Res 2019;11(2):402-405.
37. Carrillo GL, Ballard VA, Glausen T, Boone Z, Teamer J, et al.
Toxoplasma infection induces microglia-neuron contact and the loss of perisomatic inhibitory synapses.
Glia 2020;68(10):1968-1986
https://doi.org/10.1002/glia.23816



38. Machado VS, Bottari NB, Baldissera MD, Rech VC, Ianiski FR, et al. Diphenyl diselenide supplementation in infected mice by
Toxoplasma gondii: protective effect on behavior, neuromodulation and oxidative stress caused by disease.
Exp Parasitol 2016;169:51-58
https://doi.org/10.1016/j.exppara.2016.07.006


39. David CN, Frias ES, Szu JI, Vieira PA, Hubbard JA, et al. GLT-1-dependent disruption of CNS glutamate homeostasis and neuronal function by the protozoan parasite
Toxoplasma gondii.
PLoS Pathog 2016;12(6):e1005643
https://doi.org/10.1371/journal.ppat.1005643



40. Halonen SK. Immune response to
Toxoplasma gondii in the central nervous system. In Lindsay DS, Weiss LM eds, Opportunistic Infections:
Toxoplasma,
Sarcocystis, and Microsporidia. Springer. Boston, USA. 2004, pp 67-88.

41. Vargas DL, Nascimbene C, Krishnan C, Zimmerman AW, Pardo CA. Neuroglial activation and neuroinflammation in the brain of patients with autism.
Ann Neurol 2005;57(1):67-81
https://doi.org/10.1002/ana.20315


42. Croonenberghs J, Wauters A, Devreese K, Verkerk R, Scharpe S, et al. Increased serum albumin, gamma globulin, immunoglobulin IgG, and IgG2 and IgG4 in autism.
Psychol Med 2002;32(8):1457-1463
https://doi.org/10.1017/S0033291702006037


43. Spiroski M, Trajkovski V, Trajkov D, Petlichkovski A, Efinska-Mladenovska O, et al. Family analysis of immunoglobulin classes and subclasses in children with autistic disorder.
Bosn J Basic Med Sci 2009;9(4):283-289
https://doi.org/10.17305/bjbms.2009.2780



44. Heuer L, Ashwood P, Schauer J, Goines P, Krakowiak P, et al. Reduced levels of immunoglobulin in children with autism correlates with behavioral symptoms.
Autism Res 2008;1(5):275-283
https://doi.org/10.1002/aur.42



45. Warren RP, Odell JD, Warren WL, Burger RA, Maciulis A, et al. Brief report: immunoglobulin A deficiency in a subset of autistic subjects.
J Autism Dev Disord 1997;27(2):187-192
https://doi.org/10.1023/a:1025895925178


46. Trajkovski V, Ajdinski L, Spiroski M. Plasma concentration of immunoglobulin classes and subclasses in children with autism in the Republic of Macedonia: retrospective study.
Croat Med J 2004;45(6):746-749.


47. Trajkovski V, Petlichkovski A, Efinska-Mladenovska O, Trajkov D, Arsov T, et al. Higher plasma concentration of food-specific antibodies in persons with autistic disorder in comparison to their siblings.
Focus Autism Other Dev Disabil 2008;23(3):176-185
https://doi.org/10.1177/1088357608320413

48. Nayeri T, Sarvi S, Moosazadeh M, Hosseininejad Z, Sharif M, et al. Relationship between toxoplasmosis and autism: a systematic review and meta-analysis.
Microb Pathog 2020;147:104434
https://doi.org/10.1016/j.micpath.2020.104434


51. Fricker-Hidalgo H, Bailly S, Brenier-Pinchart MP, Dard C, Jean D, et al. How to estimate time of infection with
Toxoplasma gondii in pregnant women. Use of specific IgG and IgM kinetics by 7 techniques on 691 sera.
Diagn Microbiol Infect Dis 2020;96(4):114987
https://doi.org/10.1016/j.diagmicrobio.2020.114987


52. Bakunina N, Pariante CM, Zunszain PA. Immune mechanisms linked to depression via oxidative stress and neuroprogression.
Immunology 2015;144(3):365-373
https://doi.org/10.1111/imm.12443



53. Ivanova DL, Krempels R, Denton SL, Fettel KD, Saltz GM, et al. NK cells negatively regulate CD8 T cells to promote immune exhaustion and chronic
Toxoplasma gondii infection.
Front Cell Infect Microbiol 2020;10:313
https://doi.org/10.3389/fcimb.2020.00313



56. Radoń-Pokracka M, Piasecki M, Lachowska A, Baczkowski S, Spaczyńska J, et al. Assessment of the implementation of the infectious diseases screening programmes among pregnant women in the Lesser Poland region and comparison with similar programmes conducted in other European Union countries.
Ginekol Pol 2017;88(3):151-155
https://doi.org/10.5603/GP.a2017.0029


57. Schwartz CE. Aberrant tryptophan metabolism: the unifying biochemical basis for autism spectrum disorders?
Biomark Med 2014;8(3):313-315
https://doi.org/10.2217/bmm.14.11


58. Spann MN, Sourander A, Surcel HM, Hinkka-Yli-Salomäki S, Brown AS. Prenatal toxoplasmosis antibody and childhood autism.
Autism Res 2017;10(5):769-777
https://doi.org/10.1002/aur.1722


59. Kaňková S, Sulc J, Křivohlavá R, Kuběna A, Flegr J. Slower postnatal motor development in infants of mothers with latent toxoplasmosis during the first 18 months of life.
Early Hum Dev 2012;88(11):879-884
https://doi.org/10.1016/j.earlhumdev.2012.07.001


60. Berrébi A, Assouline C, Bessières MH, Lathière M, Cassaing S, et al. Long-term outcome of children with congenital toxoplasmosis.
Am J Obstet Gynecol 2010;203(6):552e1-552.e6
https://doi.org/10.1016/j.ajog.2010.06.002

62. Walther DJ, Peter JU, Bashammakh S, Hörtnagl H, Voits M, et al. Synthesis of serotonin by a second tryptophan hydroxylase isoform.
Science 2003;299(5603):76
https://doi.org/10.1126/science.1078197


63. Mahmoud ME, Fereig R, Nishikawa Y. Involvement of host defense mechanisms against
Toxoplasma gondii infection in anhedonic and despair-like behaviors in mice.
Infect Immun 2017;85(4):e00007-17
https://doi.org/10.1128/IAI.00007-17



66. Williams K, Brignell A, Randall M, Silove N, Hazell P. Selective serotonin reuptake inhibitors (SSRIs) for autism spectrum disorders (ASD).
Cochrane Database Syst Rev 2013;2013(8):CD004677. https://doi.org/10.1002/14651858.CD004677.pub3.



68. Cook EH Jr, Charak DA, Arida J, Spohn JA, Roizen NJ, et al. Depressive and obsessive-compulsive symptoms in hyperserotonemic parents of children with autistic disorder.
Psychiatry Res 1994;52(1):25-33
https://doi.org/10.1016/0165-1781(94)90117-1


69. Peng X, Brenner LA, Mathai AJ, Cook TB, Fuchs D, et al. Moderation of the relationship between
Toxoplasma gondii seropositivity and trait impulsivity in younger men by the phenylalanine-tyrosine ratio.
Psychiatry Res 2018;270:992-1000
https://doi.org/10.1016/j.psychres.2018.03.045



70. Volkow ND, Wang GJ, Fowler JS, Logan J, Schlyer D, et al. Imaging endogenous dopamine competition with [11C]raclopride in the human brain.
Synapse 1994;16(4):255-262
https://doi.org/10.1002/syn.890160402


72. Abdoli A, Dalimi A. Are there any relationships between latent
Toxoplasma gondii infection, testosterone elevation, and risk of autism spectrum disorder?
Front Behav Neurosci 2014;8:339
https://doi.org/10.3389/fnbeh.2014.00339



73. Juanah LY, Jalaludin J, Osman M, Osman ZJ. Seroprevalence of Toxoplasma gondii among schizophrenics at Hospital Kajang. Am J Infect Dis 2013;9(1):11.
74. Celik T, Kartalci S, Aytas O, Akarsu GA, Gozukara H, et al. Association between latent toxoplasmosis and clinical course of schizophrenia-continuous course of the disease is characteristic for
Toxoplasma gondii-infected patients. Folia Parasitol(Praha); 2015. 62:2015015
https://doi.org/10.14411/fp.2015.015

75. Işeri E, Güney E, Ceylan MF, Yücel A, Aral A, et al. Increased serum levels of epidermal growth factor in children with autism.
J Autism Dev Disord 2011;41(2):237-241
https://doi.org/10.1007/s10803-010-1046-3


76. Deutsch SI, Burket JA, Urbano MR, Benson AD. The α7 nicotinic acetylcholine receptor: a mediator of pathogenesis and therapeutic target in autism spectrum disorders and Down syndrome.
Biochem Pharmacol 2015;97(4):363-377
https://doi.org/10.1016/j.bcp.2015.06.005


77. Ray MA, Graham AJ, Lee M, Perry RH, Court JA, et al. Neuronal nicotinic acetylcholine receptor subunits in autism: an immunohistochemical investigation in the thalamus.
Neurobiol Dis 2005;19(3):366-377
https://doi.org/10.1016/j.nbd.2005.01.017


78. McTighe SM, Neal SJ, Lin Q, Hughes ZA, Smith DG. The BTBR mouse model of autism spectrum disorders has learning and attentional impairments and alterations in acetylcholine and kynurenic acid in prefrontal cortex.
PLoS One 2013;8(4):e62189
https://doi.org/10.1371/journal.pone.0062189



79. Bhandage AK, Barragan A. Calling in the CaValry:
Toxoplasma gondii hijacks GABAergic signaling and voltage-dependent calcium channel signaling for Trojan horse-mediated dissemination.
Front Cell Infect Microbiol 2019;9:61
https://doi.org/10.3389/fcimb.2019.00061



80. Shimmura C, Suda S, Tsuchiya KJ, Hashimoto K, Ohno K, et al. Alteration of plasma glutamate and glutamine levels in children with high-functioning autism.
PLoS One 2011;6(10):e25340
https://doi.org/10.1371/journal.pone.0025340



81. Chao HT, Chen H, Samaco RC, Xue M, Chahrour M, et al. Dysfunction in GABA signalling mediates autism-like stereotypies and Rett syndrome phenotypes.
Nature 2010;468(7321):263-269
https://doi.org/10.1038/nature09582



83. Gatkowska J, Wieczorek M, Dziadek B, Dzitko K, Dlugonska H. Sex-dependent neurotransmitter level changes in brains of
Toxoplasma gondii infected mice.
Exp Parasitol 2013;133(1):1-7
https://doi.org/10.1016/j.exppara.2012.10.005


84. Roberts CW, Cruickshank SM, Alexander J. Sex-determined resistance to
Toxoplasma gondii is associated with temporal differences in cytokine production.
Infect Immun 1995;63(7):2549-2555
https://doi.org/10.1128/iai.63.7.2549-2555.1995



85. Walker W, Roberts CW, Ferguson D, Jebbari H, Alexander J. Innate immunity to
Toxoplasma gondii is influenced by gender and is associated with differences in interleukin-12 and gamma interferon production.
Infect Immun 1997;65(3):1119-1121
https://doi.org/10.1128/IAI.65.3.1119-1121.1997



86. Lindová J, Novotná M, Havlícek J, Jozífková E, Skallová A, et al. Gender differences in behavioural changes induced by latent toxoplasmosis.
Int J Parasitol 2006;36(14):1485-1492
https://doi.org/10.1016/j.ijpara.2006.07.008


87. Lindová J, Kubena AA, Sturcová H, Krivohlavá R, Novotná M, et al. Pattern of money allocation in experimental games supports the stress hypothesis of gender differences in
Toxoplasma gondii-induced behavioural changes.
Folia Parasitol (Praha) 2010;57:136-142.


90. Kittas C, Henry L. Effect of sex hormones on the response of mice to infection with
Toxoplasma gondii.
Br J Exp Pathol 1980;61(6):590-600.


93. de Giambattista C, Ventura P, Trerotoli P, Margari F, Margari L. Sex differences in autism spectrum disorder: focus on high functioning children and adolescents.
Front Psychiatry 2021;12:539835
https://doi.org/10.3389/fpsyt.2021.539835



94. Houghton R. Utilisation and Outcomes of Treatment in Autism Spectrum Disorder [doctoral disseration]. Maastricht University. Maastricht, Netherlands. 2021,
https://doi.org/10.26481/dis.20210304rh
96. Tick B, Bolton P, Happé F, Rutter M, Rijsdijk F. Heritability of autism spectrum disorders: a meta-analysis of twin studies.
J Child Psychol Psychiatry 2016;57(5):585-595
https://doi.org/10.1111/jcpp.12499


97. Wu S, Wu F, Ding Y, Hou J, Bi J, et al. Advanced parental age and autism risk in children: a systematic review and meta-analysis.
Acta Psychiatr Scand 2017;135(1):29-41
https://doi.org/10.1111/acps.12666


98. Galeh TM, Ghazvini H, Mohammadi M, Sarvi S, Azizi S, et al. Effects of diverse types of
Toxoplasma gondii on the outcome of Alzheimer’s disease in the rat model.
Microb Pathog 2023;174:105931
https://doi.org/10.1016/j.micpath.2022.105931


99. Galeh TM, Ghazvini H, Sarvi S, Mohammadi M, Asgarian-Omran H, et al. Controversial effects of diverse types of
Toxoplasma gondii on the anxiety-like behavior and cognitive impairments in the animal model of Alzheimer’s disease.
Iran J Psychiatry Behav Sci 2022;16(3):e122961
https://doi.org/10.5812/ijpbs-122961