Sarcocystosis among Wild Captive and Zoo Animals in Malaysia
Article information
Abstract
Sarcocystis sp. infection was investigated in 20 necropsied captive wild mammals and 20 birds in 2 petting zoos in Malaysia. The gross post-mortem lesions in mammals showed marbling of the liver with uniform congestion of the intestine, and for birds, there was atrophy of the sternal muscles with hemorrhage and edema of the lungs in 2 birds. Naked eye examination was used for detection of macroscopic sarcocysts, and muscle squash for microscopic type. Only microscopically visible cysts were detected in 8 animals and species identification was not possible. Histological examination of the sections of infected skeletal muscles showed more than 5 sarcocysts in each specimen. No leukocytic infiltration was seen in affected organs. The shape of the cysts was elongated or circular, and the mean size reached 254 × 24.5 µm and the thickness of the wall up to 2.5 µm. Two stages were recognized in the cysts, the peripheral metrocytes and large numbers of crescent shaped merozoites. Out of 40 animals examined, 3 mammals and 5 birds were positive (20%). The infection rate was 15% and 25% in mammals and birds, respectively. Regarding the organs, the infection rate was 50% in the skeletal muscles followed by tongue and heart (37.5%), diaphragm (25%), and esophagus (12.5%). Further ultrastructural studies are required to identify the species of Sarcocystis that infect captive wild animals and their possible role in zoonosis.
INTRODUCTION
Sarcocystosis is a parasitic disease caused by Sarcocystis spp. which can lead to death of animals that have been highly infected. These parasites are usually found in the tissues of mammals, birds, and reptiles and have a 2-host cycle [1]. Muscular sarcocystosis has been reported in captive-born rhesus macaque [2,3], in the otter [4], in striped dolphin [5], in spotted deer, and wild red dogs [6]. The disease has also been reported in different species of birds, e.g., eagle [7], goshawk [8], wild turkey [9,10], straw-necked ibis [11], and in the lungs of parrots and pigeon [12]. In addition to muscular sarcocystosis in lizards (Hemidactylus turcicus) in Saudi Arabia [13], intestinal sarcocystosis has also been reported in the bullsnake [14].
The diagnosis of muscular sarcocystosis is usually made by the presence of cysts in organs after the animal's death, predominantly in the tongue, esophagus, diaphragm, heart, and skeletal muscles. The macroscopic cysts are easily seen with the naked eyes, but microscopic cysts can only be identified by muscle squash method, peptic digestion, and histological examination [15]. Serological tests have also been used in the diagnosis of sarcocysts infection where ELISA and indirect fluorescent antibody test (IFAT) are often used [16].
In Malaysia, sarcocystosis was reported in humans [17-24], and domestic and wild animals [25-28]. The incidence of Sarcocystis infection in wild captive zoo animals in Malaysia is relatively unknown. The aim of this study is to investigate the presence of sarcocystosis among necropsied wild captive animals in 2 zoos in Malaysia.
MATERIALS AND METHODS
Study site
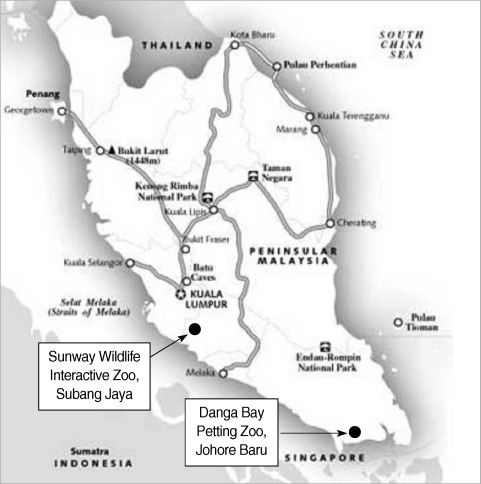
Two zoos were selected for this study; Sunway Wildlife Interactive Zoo Subang Jaya, Selangor, and Danga Bay Petting Zoo, Johore Baru, Johore, located 25 km and 225 km, respectively, from Kuala Lumpur (Fig. 1). All animals and birds that died between the periods of August to December 2008 were included. A necropsy was performed and gross abnormalities and the presence of macroscopically visible sarcocysts was documented. Samples from the tongue, esophagus, diaphragm, heart, and skeletal muscles were taken, immediately put on ice and transferred to the laboratory of the Faculty of Medicine, Universiti Teknologi MARA (UiTM), where they were stored at 4℃ for further analysis.
Microscopic examinations
Fresh muscle tissue was processed by muscle squash method as described by Latif et al. [15] and observed microscopically for intramuscular cysts. Positive samples were then fixed in 10% buffered neutral formalin and then embedded in paraffin blocks. The tissues were sectioned at 3-5 µm thickness and stained with hematoxylin and eosin. Microscopy was performed and presence or absence of the cysts was documented.
RESULTS
Of the 40 necropsied captive wild animals that were included, there were 20 mammals (12 species) and 20 birds (12 species), where 33 of the animals were from Sunway Wildlife Interactive Zoo and 7 were from Danga Bay Petting Zoo. The infection rates among each species of mammal and bird are shown in Tables 1 and 2.
Post mortem findings
The mammals showed gross marbling of the liver with uniform congestion of the intestines. The birds showed atrophy of the sternal muscles with hemorrhage and edema of the lungs. No macroscopic sarcocysts were detected in both species.
Microscopy
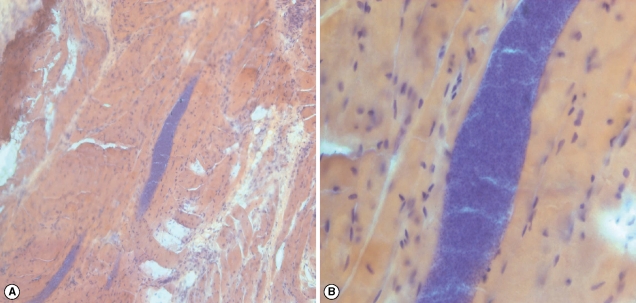
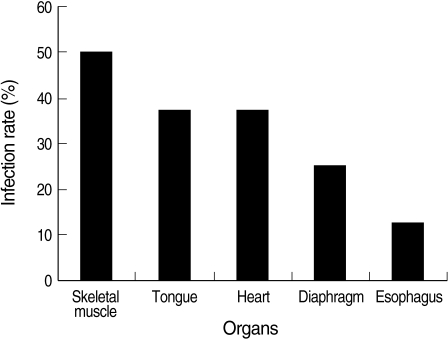
Microscopically visible cysts were detected in 8 animals (8/40; 20%). No macroscopic cysts were observed in any of the animals. The animal species, sex, and the infected organs are shown in Table 3. Histologically, the cysts were either elongated or circular with a mean measuring size of 254 × 24.5 µm. The wall was 2.5 µm in thickness. There appeared to be 2 recognizable stages; namely, the peripheral metrocytes and central crescent-shaped merozoites. Species identification was not possible. There was no leukocytic infiltration present in the adjacent muscle tissue (Fig. 2). Regarding the organs, the highest rate of infection was observed in the skeletal muscle (50%) and the lowest in the esophagus (12.5%) (Fig. 3).
DISCUSSION
In Malaysia, sarcocystosis has been reported in wild animals, such as slow loris [25], rodents [26], and long-tailed monkeys [27,28]. Worldwide, sarcocysts have been found in many types of wild animals as discussed before. No recent investigation is available for the prevalence of the infection among captive wild zoo animals in Malaysia. We have reported for the first time that sarcocystosis occurs in sun bear, swampy and agile wallabies, and guinea fowls. These animals were found to be infected at necropsy.
Results of this study showed that the infection rate of sarcocystosis was 20%, and it was 15% and 25% in mammals and birds, respectively. These animals may have acquired the infection from contaminated food and water with the feces of other wild animals (reptiles, rodents). However, it is difficult to know whether they were infected in the zoo or in the wild. The wild animals can act as definitive or intermediate hosts [1]. The definitive hosts, mostly reptiles and rodents, feed on infected preys (intermediate hosts) and develop intestinal sarcosystosis. These predators will excrete sporocysts in their feces leading to the infection of other animals that will develop muscular sarcocystosis.
Sarcocystosis was reported in psittacine birds, especially when housed outdoors or when their food is not stored correctly and contaminated with feces of infected rodents [29]. Ecco et al. [12] reported an outbreak of sarcocystosis in parrots and pigeon in Belo Horizonte Zoo in Brazil attributed to feed contaminated by feces of opossum, a common inhabitant of the forest surrounding the zoo. Also, birds pick up the sporocysts or oocysts from undigested plant materials in mammals' feces, for example, migratory Canada geese (Branta canadesis) follow cattle and feed on undigested plant materials in the cattle's feces [30]. In addition, sporocysts can also be transmitted by cockroaches or other insects. They feed on the infected animal's feces and deposit their own feces in the bird's food and water [31]. It was proven that cockatoos will eat cockroaches when added to feed. Feeding was recorded on videotape where cockatoos were seen consuming them. If the birds eat these paratenic host or food contaminated by its feces, they will develop sarcocystosis.
Sarcocystosis is often considered as incidental finding, particularly in wild-caught animals, with or without clinical symptoms [3]. In this study, a number of infected animals died with or without previous clinical signs. Most of the pathological effects are attributed to the schizont stage which causes obstruction of capillaries of the organs, especially, the lung, liver, and heart [1,10]. Pulmonary edema and hemorrhage occur, producing respiratory crisis [28]. Sudden death of these animals, without observed clinical signs, may be attributed to this condition. Ecco et al. [12] reported an outbreak of acute pulmonary sarcocystosis in parrots and pigeons with pulmonary congestion and edema, and enlarged, congested liver and spleen but still the majority of these birds were found dead without any clinical symptoms. In our study, the gross findings associated with sudden death of the animals may be attributed to sarcocystosis. Further ultrastructural studies are required to identify the species of Sarcocystis that infect captive wild animals and their possible role in zoonosis.
ACKNOWLEDGEMENTS
We would like to acknowledge the management of the Danga Bay Petting Zoo, Johore Baru, Johore and the Sunway Wildlife Interactive Zoo, Subang Jaya, Selangor for permitting us to undertake the post-mortems and the encouragement for this study. Thanks for the staff of both the zoos in assisting in the post-mortems and sample collection. We are also grateful to Prof. Dr. Harbinder Jeet Singh for his valuable revision of the manuscript and to Mrs. Norita binti Salim and Mr. Muhamad Razali for the photography. We thank Prof. Dato' Dr. Khalid Yusoff, Dean, Faculty of Medicine, UiTM, for the facilities and encouragement.