Genetic Diversity of Toxoplasma gondii Strains from Different Hosts and Geographical Regions by Sequence Analysis of GRA20 Gene
Article information
Abstract
Toxoplasma gondii is a eukaryotic parasite of the phylum Apicomplexa, which infects all warm-blood animals, including humans. In the present study, we examined sequence variation in dense granule 20 (GRA20) genes among T. gondii isolates collected from different hosts and geographical regions worldwide. The complete GRA20 genes were amplified from 16 T. gondii isolates using PCR, sequence were analyzed, and phylogenetic reconstruction was analyzed by maximum parsimony (MP) and maximum likelihood (ML) methods. The results showed that the complete GRA20 gene sequence was 1,586 bp in length among all the isolates used in this study, and the sequence variations in nucleotides were 0-7.9% among all strains. However, removing the type III strains (CTG, VEG), the sequence variations became very low, only 0-0.7%. These results indicated that the GRA20 sequence in type III was more divergence. Phylogenetic analysis of GRA20 sequences using MP and ML methods can differentiate 2 major clonal lineage types (type I and type III) into their respective clusters, indicating the GRA20 gene may represent a novel genetic marker for intraspecific phylogenetic analyses of T. gondii.
Toxoplasma gondii, one of the most successful intracellular protozoan parasites, can infect the majority of vertebrate spices including humans with a worldwide distribution [1-3], and approximately one-third of the population has been exposed to T. gondii. Normally, the infections are asymptomatic or subclinical. However, the T. gondii infection can cause abortion and stillbirth in pregnant women, and encephalitis, chorioretinitis, and systemic infections in immunocompromised individuals [2]. In animals, T. gondii can also cause abortion in livestock, especially in sheep and goats, which can spawn a great number of economic losses in livestock [3]. However, there was no effective vaccine and drugs that can help to control toxoplasmosis.
The strains of T. gondii that predominate in Europe and North America, classified into types I, II, and III, differ in a wide range of phenotypes, including virulence, persistence, migratory capacity, and how they interface with the immune response [4-6], Thus, the information of genetic diversity of T. gondii is useful for better understanding epidemiological patterns and pathogenicity, as well as exploring of new polymorphic virulence effectors.
GRA20, a novel dense granule protein, is secreted and targeted to parasitophorous vacuole membrane (PVM), which may participate in the manipulation of the host immunity [7]. Previous studies have identified the existence of polymorphisms in dense granule proteins, such as GRA15, GRA5, and GRA6 [8-10], but the sequence variation about the GRA20 gene among different T. gondii isolates is still unknown. Therefore, the objective of this study was to examine sequence diversity of GRA20 gene among T. gondii strains from different hosts and geographical regions worldwide.
In this study, a total 16 T. gondii strains from different hosts and geographic locations were used for analysis (Table 1). These T. gondii isolates have been genotyped and genomic DNA (gDNA) was prepared as described previously [11-13].
To acquire amplicons of GRA20 genes concerning different T. gondii isolates, the primers GRA20-F (5´- ATGCATAGCCGGAACTGCGTC-3´) and GRA20-R (5´- TCACGCGGGCTTTCTACGG-3´) were designed based on T. gondii ME49 strain available in ToxoDB database (TGME49_200010). All the PCR products of GRA20 genes were purified by the DNA purification kit (GenStar, Beijing, China), ligated into pMD18-T vector (TaKaRa, Dalian, China), and then transformed into JM109 competent cells (Promega, Madison, Wisconsin, USA). Subsequently, the positive colonies were screened by PCR, and then sequenced by GenScript Co., Ltd. (Nanjing, China).
The acquired GRA20 gene sequences were aligned by the Multiple Sequence Alignment Program, Clustal X 1.83 [14], and sequence variation was determined among the examined T. gondii strains. Phylogenetic reconstructions based on the complete sequences of GRA20 gene among 13 T. gondii isolates and plus the corresponding sequences of strains TgCatBr9, VEG, and ME49 available in ToxoDB (http://toxodb.org/toxo/) were carried out by 2 inference methods, maximum likelihood (ML) and maximum parsimony (MP) methods by Paup, with the sequence of Hammondia hammondi (XM_008890671.1) as the out-group. Phylograms were drawn by the Tree View program version 1.65.
In the present study, the obtained entire genomic sequences of the GRA20 gene were 1,586 bp in length in all examined isolates. According to the analysis of all the 16 GRA20 complete sequences, there were 2 extrons and 1 intron in the GRA20 gene (Table 2). The A+T content ranged from 45.0% to 45.4% in the entire sequence. There were 124 nucleotide position variations with a distribution of 57 transitions (A↔G and C↔T), 61 transversions (C↔G, T↔G, A↔C, and A↔T) in CDS, and 2 transitions (A↔G and C↔T), and 1 transversion (C↔G, T↔G, A↔C, and A↔T) in the intron (Table 2). However, when we analyzed the GRA20 sequences without type III (CTG, VEG) strains, there were 12 nucleotide variations with a distribution of 10 transitions (A↔G and C↔T), 2 transversions (C↔G, T↔G, A↔C, and A↔T) in CDS, and 2 transitions (A↔G and C↔T) and 1 transversion (C↔G, T↔G, A↔C, and A↔T) in the intron. The alignment of GRA20 gene sequences showed that sequence variation was 0-7.9% in all studied strains, while the sequence variation became 0-0.7% without the CTG and VEG strains. Phylogenetic reconstruction of all 16 T. gondii strains based on GRA20 sequence data showed that the type I and type III of T. gondii strains were clustered into respective clusters separately (Fig. 1).
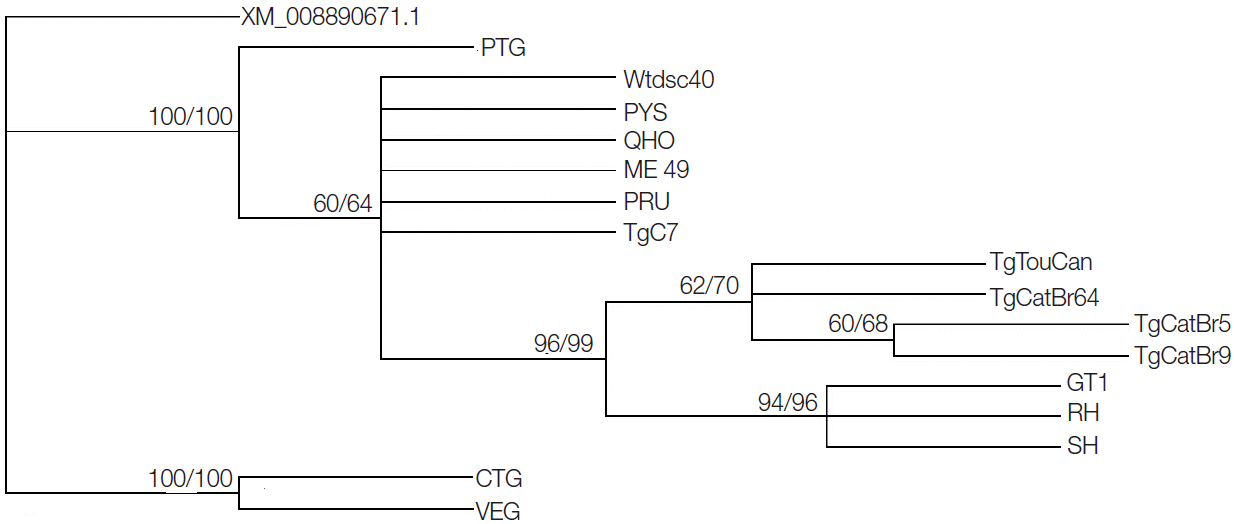
Phylogram of 16 Toxoplasma gondii isolates determined by analysis of the entire sequences of the GRA20 gene. The tree was reconstructed by maximum parsimony (MP) and maximum likelihood (ML) analyses. The numbers along branches indicate bootstrap values resulting from different analyses in the order: MP/ML.
Recently, polymorphisms in the sequences of GRA5, GRA6, GRA7, and GRA15 genes have been reported [8,9,15,16]. Among them, polymorphic dense granule proteins were widely used in typing T. gondii isolates, such as GRA6 [10]. Furthermore, polymorphic dense granule protein may have different roles in regulating the inflammatory response. For example, GRA15 in type II activate more IL-12 than type I or type III strains [8]. In this study, we found GRA20 gene was very diverse in type III, indicating the functions may be different, too. Our results were consistent with that of some previous studies using other genetic markers, such as GRA5, Rop17, and HSP60 for genotyping [9,17,18], but different to some previous studies, such as Rop38 and eIF4A [19,20].
In conclusion, the present study examined the sequences of the T. gondii GRA20 gene and revealed that it was more divergence in type III compared to other T. gondii strains, suggesting the functions of GRA20 in type III may be different from other strains. Phylogenetic analysis indicated that the GRA20 gene could distinguish the type I and type III strains, suggesting the GRA20 gene may be a novel genetic marker for studying genetic variation or the population genetic structures of T. gondii isolates.
Acknowledgements
The project support was provided by National Natural Science Foundation of China (Grant No. 31228022) and the Science Fund for Creative Research Groups of Gansu Province (Grant No. 1210RJIA006).
Notes
The authors declare that they have no competing interests.

