Discovery of Parvatrema duboisi and Parvatrema homoeotecnum (Digenea: Gymnophallidae) from Migratory Birds in Korea
Article information
Abstract
Adult worms of Parvatrema spp. (Digenea: Gymnophallidae) were found in the intestines of 2 species of migratory birds, i.e., a great knot, Calidris tenuirostris, and 2 Mongolian plovers, Charadrius mongolus, in the coastal area of Gunsan-si, Jeollabuk-do in October 2009. The recovered Parvatrema worms were 79 in total number and composed of 2 species. The worms from a great knot were 289 µm in length with the oral and ventral sucker ratio of 2 : 1. They had a single vitellarium, and their intrauterine eggs were 25.0 × 17.5 µm in size. These findings were compatible with P. duboisi (Dollfus, 1923) Bartoli, 1974 (syn. P. timondavidi Bartoli, 1963). The worms recovered from the Mongolian plovers were smaller in length than P. duboisi and had 2 vitellaria. The oral and ventral sucker ratio was 2.5 : 1, and the eggs were 17.5 × 8.8 µm in size. These worms were assigned to be P. homoeotecnum James, 1964. This is the first report on the natural final hosts of Parvatrema spp. in Korea.
Parvatrema spp. which belong to the family Gymnophallidae utilize bivalve mollusks as intermediate hosts. The unique morphologic feature of Parvatrema spp. is a large, pit-like genital pore, distinctly anterior to the ventral sucker [1]. Fourteen species of Parvatrema are so far known, among which P. polymedosa was found only as metacercaria [2-4]. In the Republic of Korea, only 2 species of Parvatrema are known, P. chaii Sohn et al., 2007 and P. duboisi (Dollfus, 1923) Bartoli, 1974 (under the name P. timondavidi) [5-7]. P. chaii was described from experimental mice fed metacercariae collected from the surf-clam, Mactra veneriformis [5]. The metacercariae of P. duboisi were found in Tapes philippinarum, a marine clam species [6], and the infection rate of the clam was 77.3% in southern coastal areas of Korea [7].
Parvatrema duboisi was originally described as Gymnophallus bursicola Odhner in Japan in 1900 [8], and redescribed as Parvatrema timondavidi Bartoli in 1963 [9]. However, P. timondavidi was considered a synonym of P. duboisi by Bartoli in 1974 [10,11]. Hence, we used the name P. duboisi rather than P. timondavidi.
The definitive hosts of gymnophallids are known to be marine and coastal birds. However, experimental infections of chicks and ducklings failed to obtain adults of P. duboisi, whereas experimental infections using mice and rats were successful to obtain adult flukes [11]. In Korea, ICR mice were used to obtain adult worms of P. duboisi and P. chaii [4,6]. Nevertheless, it is suspected that birds should play a pivotal role as the definitive host of Parvatrema spp. as seen in the case of Gymnophalloides seoi, of which the natural definitive host was the palearctic oystercatcher, Haematopus ostralegus [12]. In addition, Smith [13] suggested a strong positive association between bird abundance and the prevalence of trematode infections [13]. In the present study, we discovered the adult worms of P. duboisi and P. homoeotecnum from the intestine of migratory birds and report these findings with morphological descriptions.
In a survey of crab-mediated trematodes, a great knot (Calidris tenuirostris; Fig. 1) and 2 Mongolian plovers (Charadrius mongolus; Fig. 2) were found dead at a coastal area of Gunsan-si, Jeoallbuk-do, in October 2009. They were immediately transferred to our laboratory at 4℃ and the intestines were separated. They were opened longitudinally in saline, and the intestinal contents were examined for the presence of parasites. The trematode specimens were fixed in neutral 10% formalin, stained with carmine, and observed for species identification.

A great knot, Calidris tenuirostris, a natural definitive host for Parvatrema duboisi, from a coastal area of Gunsan-si, Jeollabuk-do.

Mongolian plover, Charadrius mongolus, a natural definitive host for Parvatrema homoeotecnum, from a coastal area of Gunsan-si, Jeollabuk-do.
Total 79 Parvatrema specimens were collected from the intestines of birds, and the taxonomic analysis revealed 2 species of Parvatrema; 27 (1 species) from the great knot and 52 (another species) from the Mongolian plover. The former species had the following morphologic characteristics (Fig. 3). Body tiny and ovoid, 289 (198-410) µm long and 162 (123-228) µm wide at the midbody. Oral sucker subterminal, large and muscular, 90 (75-120) by 93 (75-113) µm in size. Ceca short, and genital pore, a slit-like opening, was at some distance from the ventral sucker. Ventral pit absent. Ventral sucker round, located posterior to midline, 46 (30-58) by 46 (28-65) µm. Sucker ratio (OS/VS) 2 : 1. Seminal vesicle anterodextral to the ventral sucker. Two testes posterolateral to the ventral sucker, right testis 28 (20-35) by 20 (15-25) µm. Ovary anterior to the right testis and in the same level with ventral sucker, 32 (25-38) by 25 (20-33) µm. Vitelliaria was present as a single mass. The size of intrauterine eggs was 25.0 by 17.5 µm. From these findings, the worms recovered from the great knot were proved to be P. duboisi.
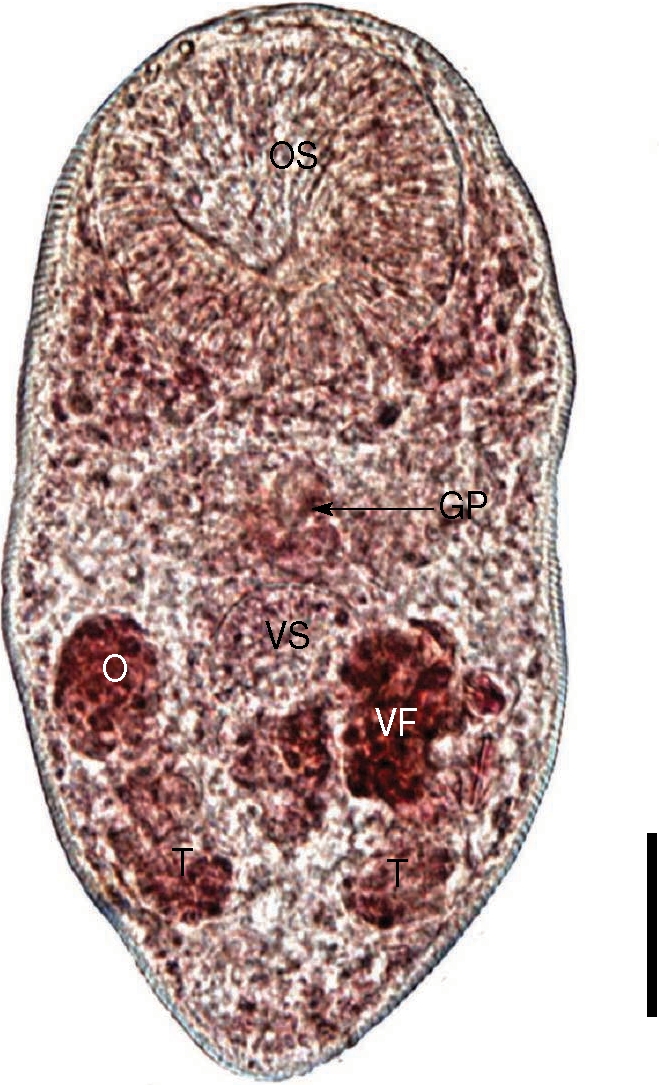
An adult worm of Parvatrema duboisi from the intestine of a great knot. Note that the vitellarine follicle (VF) is present as a single mass. OS, oral sucker; VS, ventral sucker; GP, genital pore; O, ovary; T, testis. Bar = 30 µm.
The worms from the Mongolian plover were smaller than P. duboisi in length and width, 206 (175-223) µm and 131 (115-150) µm, respectively (Fig. 4). Prepharynx not seen. Pharynx well developed, muscular. Oral sucker 85 (78-90) by 85 (75-95) µm, ventral sucker 33 (28-38) by 34 (25-38) µm. Sucker ratio (OS/VS) 2.5 : 1. Ventral sucker was located at the same level of testes. Right testis 36 (30-45) by 25 (23-35) µm, ovary 32 (25-38) by 19 (15-30) µm. Genital pore located between oral sucker and ventral one, more close to the latter. Vitellarium was paired into two lobes, overlapping the anterior border of ventral sucker. The intrauterine eggs were 17.5 × 8.8 µm in size. These findings were enough to describe them as P. homoeotecnum.
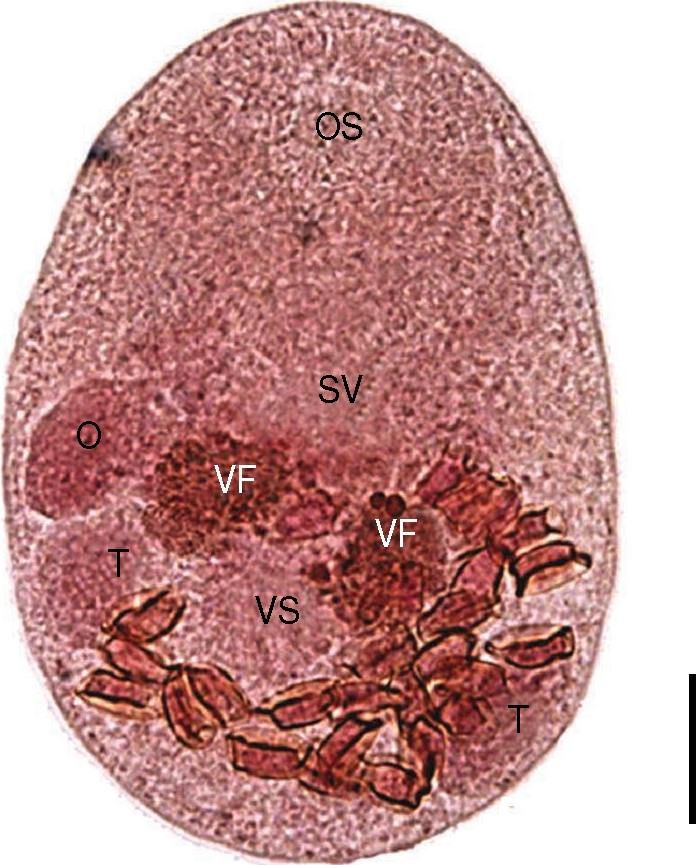
An adult worm of Parvatrema homoeotecnum from the intestine of a Mongolian plover. The vitellarine follicle (VF) is present as 2 masses. Genital pore is not seen because of overlapping with the seminal vesicle (SV). OS, oral sucker; VS, ventral sucker; O, ovary; T, testis. Bar = 30 µm.
From this study, it has been shown that the great knot and Mongolian plover serve as the natural final hosts of P. duboisi and P. homoeotecnum, respectively. Since P. duboisi is transmitted by T. philippinarum [6], the migratory birds might be infected from eating these bivalves in Korea or other countries, from which the birds migrated. During the non-breeding season, the Mongolian plover takes insects, crustaceans, including crabs and amphipods, and mollusks, particularly bivalves [14]. The great knot is also known to feed on bivalves from intertidal mudflats, as well as gastropods, crustaceans, annelid worms, and echinoderms during the non-breeding season [15]. From this viewpoint, it seems natural that these migratory birds serve as the definitive hosts for Parvatrema spp. in Korea. In addition, the metacercariae of P. duboisi had been reported from the razor clam (Sinnovacula constricta), caught at Gyehwa-myon, Buan-gun, and Jeollabuk-do [16]. Since the adult worms were not obtained at that time, further research is needed to confirm the razor clam as an intermediate host for P. duboisi.
In our study, the worms from the great knot could be identified as P. duboisi. Other species, including P. affinis, P. margaritense, P. borinquenae, and P. ovoplenum, are much smaller than our specimens [2-4]. While the vitellaria are paired in P. margaritense, P. homoeotecnum, P. borealis, and P. obscuru [2-5], the vitellarium is single in the present worms. The ratio of the oral to ventral sucker was 2 : 1, similar to P. duboisi, P. affinis, P. borealis, and P. obscurum [2,11]. These findings were compatible with P. duboisi. The size of intrauterine eggs was slightly smaller than those described by Yu et al. [6], but similar to those described by Yanagida et al. [11].
On the other hand, the worms from the Mongolian plover were distinguished from P. duboisi in many aspects. Considering the paired vitellaria and sucker ratio of 2.5 : 1, these worms could be assigned as P. homoeotecnum. The intermediate host of P. homoeotecnum is known to be the gastropod (Littorina saxatilis), and the final host was shown to be oystercatchers (Haematopus ostralegus occidentalis) [2]. Since 10,000-20,000 worms occurred in each oystercatcher [2], oystercatchers might be a more suitable host than the Mongolian plover. The relationship between birds and trematode infections has not been frequently reported in Korea. Considering the pivotal role of birds in the transmission of parasitic diseases, more attention should be paid to this subject.