Abstract
Adult worms of Parvatrema spp. (Digenea: Gymnophallidae) were found in the intestines of 2 species of migratory birds, i.e., a great knot, Calidris tenuirostris, and 2 Mongolian plovers, Charadrius mongolus, in the coastal area of Gunsan-si, Jeollabuk-do in October 2009. The recovered Parvatrema worms were 79 in total number and composed of 2 species. The worms from a great knot were 289 µm in length with the oral and ventral sucker ratio of 2 : 1. They had a single vitellarium, and their intrauterine eggs were 25.0 × 17.5 µm in size. These findings were compatible with P. duboisi (Dollfus, 1923) Bartoli, 1974 (syn. P. timondavidi Bartoli, 1963). The worms recovered from the Mongolian plovers were smaller in length than P. duboisi and had 2 vitellaria. The oral and ventral sucker ratio was 2.5 : 1, and the eggs were 17.5 × 8.8 µm in size. These worms were assigned to be P. homoeotecnum James, 1964. This is the first report on the natural final hosts of Parvatrema spp. in Korea.
-
Key words: Parvatrema duboisi, Parvatrema homoeotecnum, Gymnophallidae, Mongolian plover, great knot
Parvatrema spp. which belong to the family Gymnophallidae utilize bivalve mollusks as intermediate hosts. The unique morphologic feature of
Parvatrema spp. is a large, pit-like genital pore, distinctly anterior to the ventral sucker [
1]. Fourteen species of
Parvatrema are so far known, among which
P. polymedosa was found only as metacercaria [
2-
4]. In the Republic of Korea, only 2 species of
Parvatrema are known,
P. chaii Sohn et al., 2007 and
P. duboisi (Dollfus, 1923) Bartoli, 1974 (under the name
P. timondavidi) [
5-
7].
P. chaii was described from experimental mice fed metacercariae collected from the surf-clam,
Mactra veneriformis [
5]. The metacercariae of
P. duboisi were found in
Tapes philippinarum, a marine clam species [
6], and the infection rate of the clam was 77.3% in southern coastal areas of Korea [
7].
Parvatrema duboisi was originally described as
Gymnophallus bursicola Odhner in Japan in 1900 [
8], and redescribed as
Parvatrema timondavidi Bartoli in 1963 [
9]. However,
P. timondavidi was considered a synonym of
P. duboisi by Bartoli in 1974 [
10,
11]. Hence, we used the name
P. duboisi rather than
P. timondavidi.
The definitive hosts of gymnophallids are known to be marine and coastal birds. However, experimental infections of chicks and ducklings failed to obtain adults of
P. duboisi, whereas experimental infections using mice and rats were successful to obtain adult flukes [
11]. In Korea, ICR mice were used to obtain adult worms of
P. duboisi and
P. chaii [
4,
6]. Nevertheless, it is suspected that birds should play a pivotal role as the definitive host of
Parvatrema spp. as seen in the case of
Gymnophalloides seoi, of which the natural definitive host was the palearctic oystercatcher,
Haematopus ostralegus [
12]. In addition, Smith [
13] suggested a strong positive association between bird abundance and the prevalence of trematode infections [
13]. In the present study, we discovered the adult worms of
P. duboisi and
P. homoeotecnum from the intestine of migratory birds and report these findings with morphological descriptions.
In a survey of crab-mediated trematodes, a great knot (
Calidris tenuirostris;
Fig. 1) and 2 Mongolian plovers (
Charadrius mongolus;
Fig. 2) were found dead at a coastal area of Gunsan-si, Jeoallbuk-do, in October 2009. They were immediately transferred to our laboratory at 4℃ and the intestines were separated. They were opened longitudinally in saline, and the intestinal contents were examined for the presence of parasites. The trematode specimens were fixed in neutral 10% formalin, stained with carmine, and observed for species identification.
Total 79
Parvatrema specimens were collected from the intestines of birds, and the taxonomic analysis revealed 2 species of
Parvatrema; 27 (1 species) from the great knot and 52 (another species) from the Mongolian plover. The former species had the following morphologic characteristics (
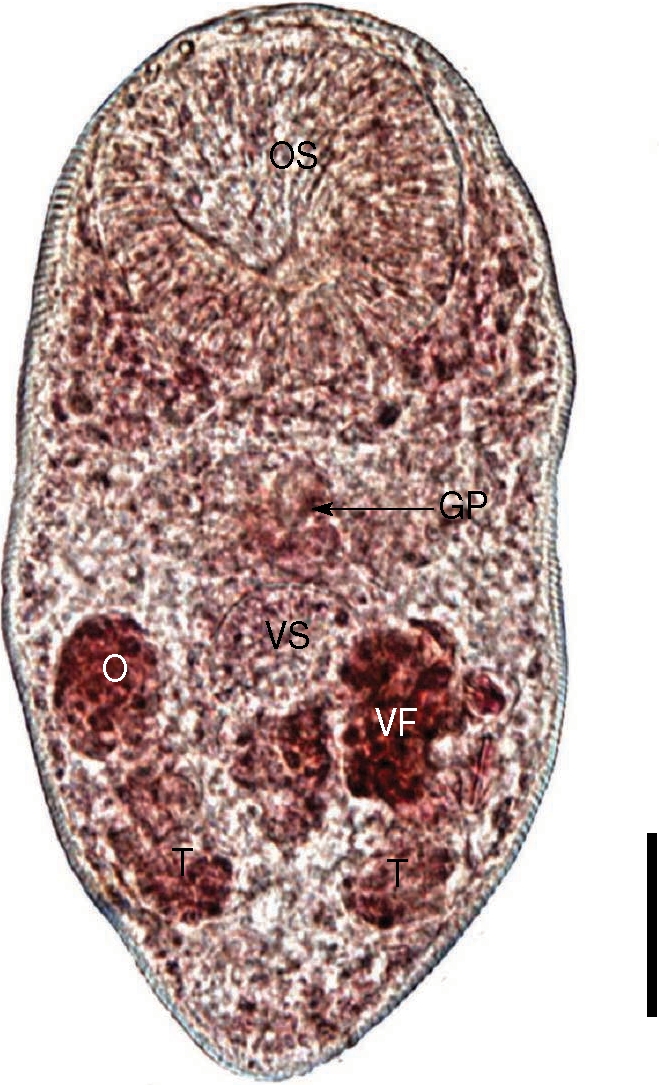
Fig. 3). Body tiny and ovoid, 289 (198-410) µm long and 162 (123-228) µm wide at the midbody. Oral sucker subterminal, large and muscular, 90 (75-120) by 93 (75-113) µm in size. Ceca short, and genital pore, a slit-like opening, was at some distance from the ventral sucker. Ventral pit absent. Ventral sucker round, located posterior to midline, 46 (30-58) by 46 (28-65) µm. Sucker ratio (OS/VS) 2 : 1. Seminal vesicle anterodextral to the ventral sucker. Two testes posterolateral to the ventral sucker, right testis 28 (20-35) by 20 (15-25) µm. Ovary anterior to the right testis and in the same level with ventral sucker, 32 (25-38) by 25 (20-33) µm. Vitelliaria was present as a single mass. The size of intrauterine eggs was 25.0 by 17.5 µm. From these findings, the worms recovered from the great knot were proved to be
P. duboisi.
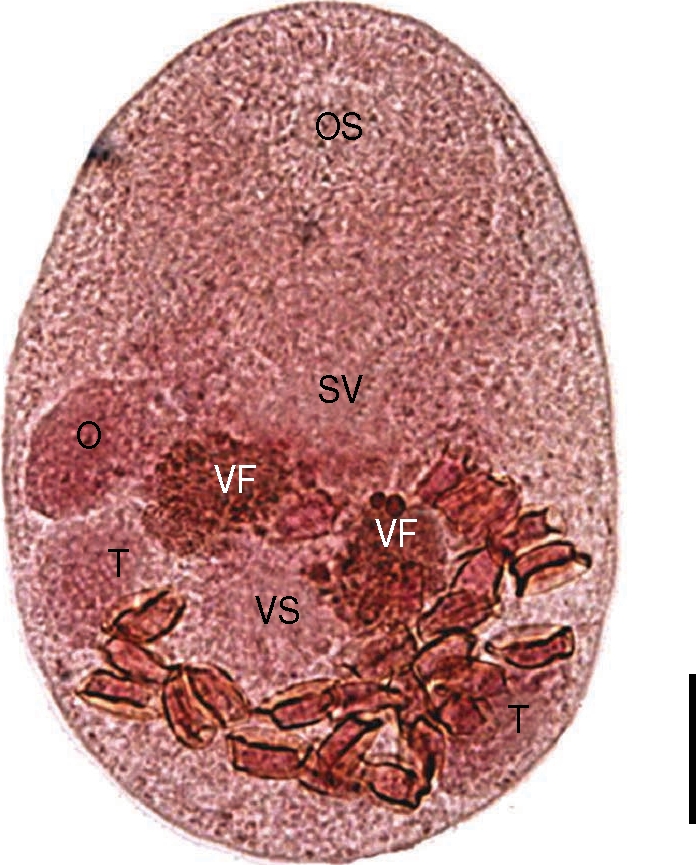
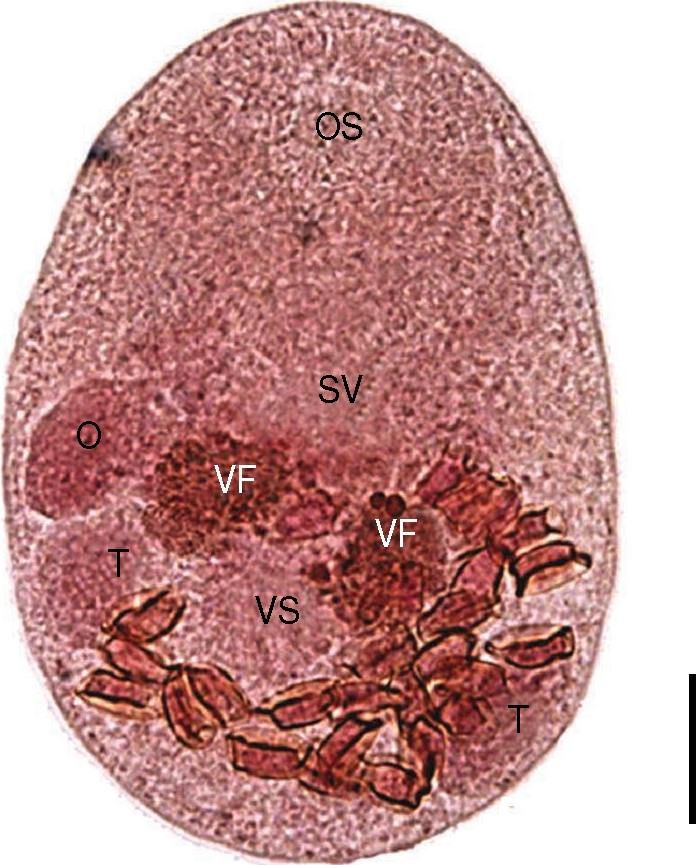
The worms from the Mongolian plover were smaller than
P. duboisi in length and width, 206 (175-223) µm and 131 (115-150) µm, respectively (
Fig. 4). Prepharynx not seen. Pharynx well developed, muscular. Oral sucker 85 (78-90) by 85 (75-95) µm, ventral sucker 33 (28-38) by 34 (25-38) µm. Sucker ratio (OS/VS) 2.5 : 1. Ventral sucker was located at the same level of testes. Right testis 36 (30-45) by 25 (23-35) µm, ovary 32 (25-38) by 19 (15-30) µm. Genital pore located between oral sucker and ventral one, more close to the latter. Vitellarium was paired into two lobes, overlapping the anterior border of ventral sucker. The intrauterine eggs were 17.5 × 8.8 µm in size. These findings were enough to describe them as
P. homoeotecnum.
From this study, it has been shown that the great knot and Mongolian plover serve as the natural final hosts of
P. duboisi and
P. homoeotecnum, respectively. Since
P. duboisi is transmitted by
T. philippinarum [
6], the migratory birds might be infected from eating these bivalves in Korea or other countries, from which the birds migrated. During the non-breeding season, the Mongolian plover takes insects, crustaceans, including crabs and amphipods, and mollusks, particularly bivalves [
14]. The great knot is also known to feed on bivalves from intertidal mudflats, as well as gastropods, crustaceans, annelid worms, and echinoderms during the non-breeding season [
15]. From this viewpoint, it seems natural that these migratory birds serve as the definitive hosts for
Parvatrema spp. in Korea. In addition, the metacercariae of
P. duboisi had been reported from the razor clam (
Sinnovacula constricta), caught at Gyehwa-myon, Buan-gun, and Jeollabuk-do [
16]. Since the adult worms were not obtained at that time, further research is needed to confirm the razor clam as an intermediate host for
P. duboisi.
In our study, the worms from the great knot could be identified as
P. duboisi. Other species, including
P. affinis,
P. margaritense,
P. borinquenae, and
P. ovoplenum, are much smaller than our specimens [
2-
4]. While the vitellaria are paired in
P. margaritense,
P. homoeotecnum,
P. borealis, and
P. obscuru [
2-
5], the vitellarium is single in the present worms. The ratio of the oral to ventral sucker was 2 : 1, similar to
P. duboisi,
P. affinis,
P. borealis, and
P. obscurum [
2,
11]. These findings were compatible with
P. duboisi. The size of intrauterine eggs was slightly smaller than those described by Yu et al. [
6], but similar to those described by Yanagida et al. [
11].
On the other hand, the worms from the Mongolian plover were distinguished from
P. duboisi in many aspects. Considering the paired vitellaria and sucker ratio of 2.5 : 1, these worms could be assigned as
P. homoeotecnum. The intermediate host of
P. homoeotecnum is known to be the gastropod (
Littorina saxatilis), and the final host was shown to be oystercatchers (
Haematopus ostralegus occidentalis) [
2]. Since 10,000-20,000 worms occurred in each oystercatcher [
2], oystercatchers might be a more suitable host than the Mongolian plover. The relationship between birds and trematode infections has not been frequently reported in Korea. Considering the pivotal role of birds in the transmission of parasitic diseases, more attention should be paid to this subject.
References
- 1. Cable RM. The life cycle of Parvatrema boringquenae gen. et sp. nov. (Trematoda: Digenea) and the systematic position of the subfamily Gymnophallinae. J Parasitol 1953;39:408-421.
- 2. James BL. The life cycle of Parvatrema homoeotecnum sp. nov. (Trematoda: Digenea) and a review of the family Gymnophallidae Morozov, 1955. Parasitology 1964;54:1-41.
- 3. Ching HL. Four new gymnophallid digeneans from rice rats, willets, and mollusks in Florida. J Parasitol 1995;81:924-928.
- 4. Galaktionov KV, Irwin SWB, Shaville DH. One of the most complex life-cycles among trematodes: a description of Parvatrema margaritense (Ching, 1982) n. comb. (Gymnophallidae) possessing parthenogenetic metacercariae. Parasitology 2006;132:733-746.
- 5. Sohn WM, Na BK, Ryang YS, Ching HL, Lee SH. Parvatrema chaii n. sp. (Digenea: Gymanophallidae) from mice experimentally infected with metacercariae collected from surf-clam, Mactra veneriformis. Korean J Parasitol 2007;45:115-120.
- 6. Yu JR, Chai JY, Lee SH. Parvatrema timondavidi (Digenea: Gymnophallidae) transmitted by a clam, Tapes philippinarum, in Korea. Korean J Parasitol 1993;31:7-12.
- 7. Sohn WM, Chai JY, Lee SH. Infection status of Tapes philippinarum collected from southern coastal areas of Korea with Parvatrema spp. (Digenea: Gymnophallidae) metacercariae. Korean J Parasitol 1996;34:273-277.
- 8. Ogada T. On the morphology, ecology and life history of an agamodistome parasitic in a bivalve, Paphia (Ruditapes) philippinarum (Adams et Reeve). Sci Rep Tokyo Bunrika Daigaku, Sec B 1944;7:1-24.
- 9. Endo T, Hoshina T. Redescription and identification of a gymnophallid trematode in a brackish water clam, Tapes (Ruditapes) philippinarum. Jpn J Parasitol 1974;23:73-77.
- 10. Bartoli P. Recherches sur le Gmnophallidae F. N. Morozov, 1955 (Digenea) parasites d'oiseaux des cotes de Camarague: systematigue, biologie et ecologie. 1974, Universite de Droit, d'Economie et des Sciences d'Aix-Marseille; These.
- 11. Yanagida T, Shirakashi S, Iwaki T, Ikushima N, Ogawa K. Gymnophallid digenean Parvatrema duboisi uses Manila clam as the first and second intermediate host. Parasitol Int 2009;58:308-310.
- 12. Ryang YS, Yoo JC, Lee SH, Chai JY. The palearctic oystercatcher Haematopus ostralegus, a natural definitive host for Gymnophalloides seoi. J Parasitol 2000;86:418-419.
- 13. Smith NF. Associations between shorebird abundance and parasites in the sand crab, Emerita analoga, along the California coast. J Parasitol 2007;93:265-273.
- 14. Domm S, Recher HF. The birds of One Tree Island with notes on their yearly cycle and feeding ecology. Sunbird 1973;4:63-86.
- 15. Tomkovitch PS. A third report on the biology of the great knot Calidris tenuirostris on the breeding grounds. Stilt 1996;28:43-45.
- 16. Kim YG, Yun KS. Trematodes larva in 3 species of bivalves (Corbicula japonica, Sinnovacula consticta and Ruditapes philippinarum). J Fish Pathol 2003;16:203-213.
Fig. 1A great knot, Calidris tenuirostris, a natural definitive host for Parvatrema duboisi, from a coastal area of Gunsan-si, Jeollabuk-do.

Fig. 2Mongolian plover, Charadrius mongolus, a natural definitive host for Parvatrema homoeotecnum, from a coastal area of Gunsan-si, Jeollabuk-do.

Fig. 3An adult worm of Parvatrema duboisi from the intestine of a great knot. Note that the vitellarine follicle (VF) is present as a single mass. OS, oral sucker; VS, ventral sucker; GP, genital pore; O, ovary; T, testis. Bar = 30 µm.
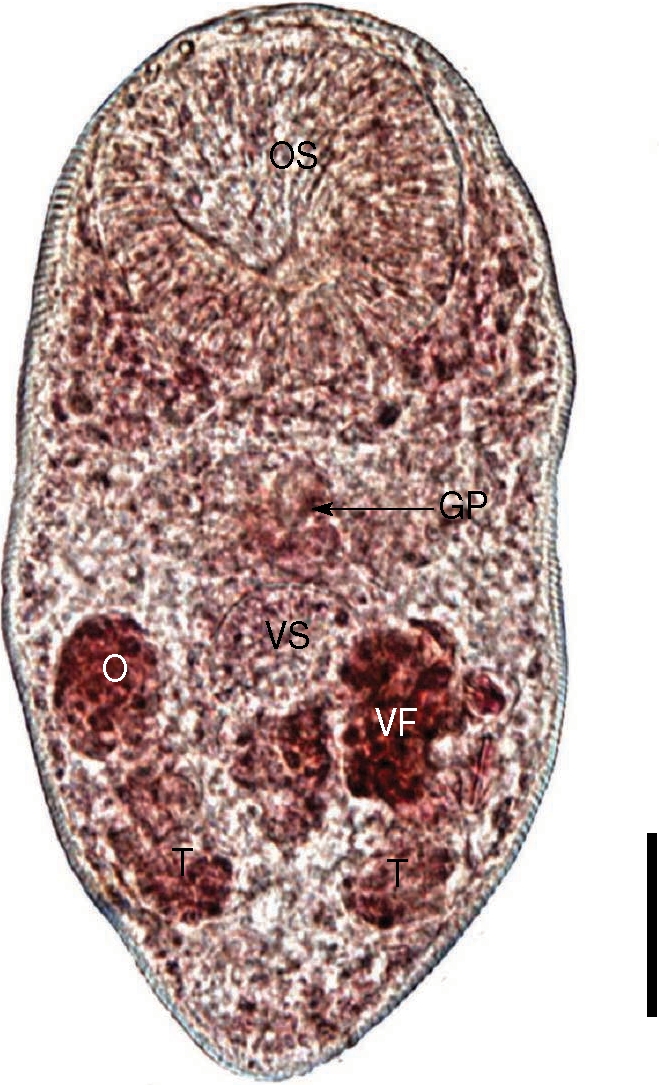
Fig. 4An adult worm of Parvatrema homoeotecnum from the intestine of a Mongolian plover. The vitellarine follicle (VF) is present as 2 masses. Genital pore is not seen because of overlapping with the seminal vesicle (SV). OS, oral sucker; VS, ventral sucker; O, ovary; T, testis. Bar = 30 µm.
Citations
Citations to this article as recorded by

- Parvatrema spp. (Digenea, Gymnophallidae) with parthenogenetic metacercariae: diversity, distribution and host specificity in the palaearctic
Kirill V. Galaktionov, Anna Gonchar, Daria Postanogova, Aleksei Miroliubov, Semen Yu. Bodrov
International Journal for Parasitology.2024;[Epub] CrossRef - Parvatrema duboisi (Digenea: Gymnophallidae) Life Cycle Stages in Manila Clams, Ruditapes philippinarum, from Aphae-do (Island), Shinan-gun, Korea
Bong-Kwang Jung, Taehee Chang, Hyejoo Shin, Seungwan Ryoo, Sooji Hong, Jeonggyu Lee, Hyemi Song, Jaeeun Cho, Deok-Gyu Kim, Hojong Jun, Min-Jae Kim, Eun Jeong Won, Eun-Taek Han, Eun-Hee Shin, Jong-Yil Chai
The Korean Journal of Parasitology.2021; 59(1): 83. CrossRef - Molecular data reshape our understanding of the life cycles of three digeneans (Monorchiidae and Gymnophallidae) infecting the bivalve, Donax variabilis: it’s just a facultative host!
Kristina M. Hill-Spanik, Claudia Sams, Vincent A. Connors, Tessa Bricker, Isaure de Buron
Parasite.2021; 28: 34. CrossRef - Morphological and Molecular Confirmation of Parvatrema duboisi Metacercariae in the Manila Clam Ruditapes philippinarum from Gochang-gun, Korea
Taehee Chang, Bong-Kwang Jung, Hyejoo Shin, Sooji Hong, Jeonggyu Lee, Deok-Gyu Kim, Laddawan Patarwut, Woon-Mok Sohn, Jong-Yil Chai
The Korean Journal of Parasitology.2020; 58(1): 87. CrossRef - Infections with Digenetic Trematode Metacercariae in Freshwater Fishes from Two Visiting Sites of Migratory Birds in Gyeongsangnam-do, Republic of Korea
Woon-Mok Sohn, Byoung-Kuk Na
The Korean Journal of Parasitology.2019; 57(3): 273. CrossRef - Fossils of parasites: what can the fossil record tell us about the evolution of parasitism?
Tommy L. F. Leung
Biological Reviews.2017; 92(1): 410. CrossRef - New Record of Schistorophus cirripedesmi (Nematoda: Acuariidae) from a Bar-Tailed Godwit, Limosa lapponica baueri (Charadriformes: Scolopacidae) in Korea
Seongjun Choe, Hyun Kim, Junsik Lim, Dongmin Lee, Hansol Park, Hyeong-Kyu Jeon, Heejong Kim, Youngjun Kim, Keeseon S. Eom
The Korean Journal of Parasitology.2016; 54(3): 349. CrossRef - Seasonality and host–parasite interrelationship of Mytilus galloprovincialis parasites in Turkish Black Sea coasts
Ahmet Özer, Sevilay Güneydağ
Journal of the Marine Biological Association of the United Kingdom.2015; 95(8): 1591. CrossRef - First report of Urosporidium sp., a haplosporidian hyperparasite infecting digenean trematode Parvatrema duboisi in Manila clam, Ruditapes philippinarum on the west coast of Korea
Thanh Cuong Le, Hyun-Sil Kang, Hyun-Ki Hong, Kwang-Jae Park, Kwang-Sik Choi
Journal of Invertebrate Pathology.2015; 130: 141. CrossRef - Discovery ofMaritrema obstipum(Digenea: Microphallidae) from Migratory Birds in Korea
Ok-Sik Chung, Woon-Mok Sohn, Jong-Yil Chai, Min Seo, Hye-Jung Lee
The Korean Journal of Parasitology.2011; 49(4): 457. CrossRef