Prostaglandin synthase activity of sigma- and mu-class glutathione transferases in a parasitic trematode, Clonorchis sinensis
Article information
Abstract
Sigma-class glutathione transferase (GST) proteins with dual GST and prostaglandin synthase (PGS) activities play a crucial role in the establishment of Clonorchis sinensis infection. Herein, we analyzed the structural and enzymatic properties of sigma-class GST (CsGST-σ) proteins to obtain insight into their antioxidant and immunomodulatory functions in comparison with mu-class GST (CsGST-μ) proteins. CsGST-σ proteins conserved characteristic structures, which had been described in mammalian hematopoietic prostaglandin D2 synthases. Recombinant forms of these CsGST-σ and CsGST-μ proteins expressed in Escherichia coli exhibited considerable degrees of GST and PGS activities with substantially different specific activities. All recombinant proteins displayed higher affinities toward prostaglandin H2 (PGS substrate; average Km of 30.7 and 3.0 μm for prostaglandin D2 [PGDS] and E2 synthase [PGES], respectively) than those toward CDNB (GST substrate; average Km of 1,205.1 μm). Furthermore, the catalytic efficiency (Kcat/Km) of the PGDS/PGES activity was higher than that of GST activity (average Kcat/Km of 3.1, 0.7, and 7.0×10−3 s−1μm−1 for PGDS, PGES, and GST, respectively). Our data strongly suggest that the C. sinensis sigma- and mu-class GST proteins are deeply involved in regulating host immune responses by generating PGD2 and PGE2 in addition to their roles in general detoxification.
Introduction
Glutathione transferase (GST) catalyzes the conjugation of glutathione (GSH) thiolate anion with various xenobiotics or intracellularly generated cytotoxic substances. The solubilized GSH conjugates of these toxic molecules are then removed by relevant transporter proteins present in plasma membranes [1]. In addition to the GSH-tagging activity, several GSTs function as peroxidases, maleylacetoacetate isomerases, or thiol transferases [2–4]. Interestingly, the detoxifying enzymes are involved in the modulation of immune responses by mediating prostaglandin (PG) and leukotriene syntheses [5]. Mammalian GSTs are classified into multiple classes including alpha, mu, pi, omega, sigma, theta, and zeta, based on their amino acid (aa) conservation patterns and substrate/inhibitor specificity, while a series of proteins show mosaic patterns in their enzymatic properties [6]. Homologs of the diverse GST-class members have been identified in parasitic trematodes [7–9].
Prostaglandins are oxygenated eicosanoids that participate in several physiological processes, including inflammatory immune responses [10]. Prostaglandins are produced from polyunsaturated fatty acids, mostly arachidonic acids, via cyclooxygenase (formation of PGH2) and subsequent PG species-specific PG synthase (PGS) activities [10]. Of the multiple PG species, such as PGI2, PGF2α, PGD2, and PGE2, PGD2 plays a principal role in regulating inflammatory responses by recruiting Th2 cells, eosinophils, and basophils and thereby controls the pathogenesis of allergic diseases and vasodilatation [11]. In mammals, generation of PGD2 from PGH2 is catalyzed by 2 distinct enzymes, GSH-independent lipocalin-type PGD2 synthase (L-PGDS) and GSH-dependent hematopoietic PGDS (HPGDS) [12]. The mammalian HPGDS shares structural properties with the sigma-class GSTs [5,13]. Levels of immunomodulatory PGs are frequently elevated in vertebrates during infections with parasitic helminths [14]. Therefore, helminth parasites are likely to express a protein(s) either exhibiting PGS activity or that stimulates host cells to synthesize PGs [14]. Sigma-class GSTs characterized in Onchocerca volvulus, Schistosoma haematobium, and Fasciola hepatica can be considered as the genuine homologs or, at least, functional equivalents of the mammalian HPGDS [15–17].
Proteins secreted into host environments or localized in parasite teguments may constitute the first line of defense to protect invading worms against host immune factors. Various GST-class members are commonly identified in the secretory-excretory products (ESPs) of trematode parasites such as F. hepatica [18], Opisthorchis viverrini [19], and Clonorchis sinensis [9]. Of the secreted proteins, the sigma-class GST proteins comprise the second most abundant component of the C. sinensis ESP [9]. The F. hepatica ESP also contains a protein categorized into this class, although the relative concentration was much lower than that of the C. sinensis orthologs [20,21]. Consequently, we hypothesized that the secreted sigma-class GST plays an important role in relation to the modulation of host immune response by providing PGS activities during trematode infections.
Clonorchis sinensis is a digenean trematode that infects the bile ducts of piscivorous mammals, including humans. Humans are usually infected with the liver fluke by eating raw or undercooked freshwater fish harboring metacercaria (food-borne trematodiasis). Clonorchiasis is highly prevalent in several Asian countries including Korea, China, and Vietnam, and imposes substantial socioeconomic and public health burdens in these endemic areas [22]. Patients with heavy infection exhibit clinical symptoms such as fever, right upper-quadrant pain, and intermittent colic pain, while those with light infection are generally asymptomatic. However, chronic infection with C. sinensis can cause fibrosis of the bile ducts, destruction of surrounding liver parenchyma, and recurrent pyogenic cholangitis. Furthermore, the long-standing clonorchiasis is significantly associated with the development of cholangiocarcinoma, a biliary ductal carcinoma that has a poor prognosis [23].
In addition to the repeated ingestion of C. sinensis metacercariae, transition of host immune response from type 1 to type 2, which can be triggered by the parasite factors, helps establish chronic clonorchiasis by generating parasite-friendly environments [24]. Several Clonorchis proteins with spatiotemporal expression profiles predominantly found in the adult stage have been identified as the plausible immunomodulators [24]. Lipid mediators including PGs and leukotrienes generated by host and/or parasite enzymes also exert a profound effect during the initiation phase of type 2 immune responses [25]. Herein, we investigated the enzymatic properties of sigma- and mu-class GSTs detected in C. sinensis ESP to assess their potential as the PG-generating immunomodulator.
Materials and Methods
Retrieval of GST sequences
Genomic and proteomic sequences of C. sinensis deposited in GenBank (http://www.ncbi.nlm.nih.gov/genbank/) were screened via BLAST programs using the aa sequences of the previously described Fasciola and Clonorchis sigma-class GST proteins [8,9]. Orthologous genes/proteins were also obtained for other trematode parasites, such as O. viverrini and Schistosoma spp., from GenBank and GeneDB (http://www.genedb.org/Homepage). The presence of the GST domain in each of the isolated protein sequences was confirmed using SMART (http://smart.embl.de/). GST sequences homologous to those of the mu-class GST were also isolated for C. sinensis.
Structural and evolutionary characterization
The aa sequences of CsGST proteins were aligned using MUSCLE (https://www.ebi.ac.uk/jdispatcher/msa/muscle/) together with those of sigma-class GSTs from other trematodes and from Homo sapiens and sea squirt (Ciona intestinalis). Sequences of mu-GSTs of C. sinensis and O. viverrini, and zeta-GST of C. sinensis were also included in the alignment. The sequence alignment was used in the construction of a maximum likelihood phylogenetic tree with PhyML 3.1 (Jones–Taylor–Thornton model with frequencies for aa substitutions, gamma distributed rates among sites, and pairwise deletion of missing data). The significance of branching nodes was estimated by the nonparametric Shimodaira–Hasegawa-like approximate likelihood ratio test provided by PhyML with 1,000 replicates of the initial input alignment. The phylogenetic tree was displayed using TreeView.
The secondary and tertiary structures of CsGSTs were predicted using I-TASSER (ver. 5.0; http://zhanglab.ccmb.med.umich.edu/I-TASSER/), which combines threading, ab initio modeling, and structural refinement. The template modeling-score (TM-score) and root mean square deviation were calculated between Clonorchis proteins and reference models and were then used to evaluate the quality of the predicted tertiary structure(s). Resultant tertiary structures were visualized using PyMol (DeLano Scientific, San Carlos, CA, USA).
Gene cloning and generation of recombinant proteins
Total RNA was extracted from adult C. sinensis worms using QIAzol lysis reagent and an RNeasy Mini kit (Qiagen, Hilden, Germany). After treatment with RNase-free DNase (New England Biolabs, Ipswich, MA, USA), the RNA sample was used to synthesize cDNA with the iScript cDNA synthesis kit (Bio-Rad, Munich, Germany). Complete open reading frames (ORFs) of the C. sinensis GST genes were amplified from the cDNA using gene-specific primers containing BamH I/Not I or EcoR I/Xho I sites (Supplementary Table S1). After digestion with the corresponding endonucleases, PCR products were ligated into the pET-28a vector (Novagen, Madison, WI, USA). Plasmid constructs were transformed into E. coli DH5α cells and their expression fidelity was confirmed via sequencing. Validated plasmids were subsequently introduced into Eschrichia coli BL21 (DE3) cells. Expression of the heterologous genes was induced in Luria–Bertani medium containing 0.5 mm isopropyl-β-D-thiogalactopyranoside for 4 h at 37oC. Bacterial cells were sonicated, and the recombinant GST proteins (rCsGSTs) were purified under native conditions via nickel-nitrilotriacetic acid agarose chromatography (Qiagen, Valencia, CA, USA). Eluents were examined with 12% SDS-PAGE under reducing conditions and quantified using the Pierce BCA protein assay kit (Thermo Fisher Scientific, Waltham, MA, USA).
Assay of enzymatic activities
Purified recombinant GST protein solutions were dialyzed against phosphate buffer (100 mm, pH 7.0) for 48 h at 4oC with 3 successive buffer changes and then used in the determination of GST and PGDS activities. The GST activity was assayed using a GST assay kit (Sigma, St. Louis, MO, USA) following the manufacturer’s instruction and using 1-chloro-2,4-dinitrobenzene (CDNB) as a GST-specific substrate. The PGDS and PGE2 synthase (PGES) activities were similarly examined using PGDS and PGES ELISA kits, respectively, where PGH2 was used as a substrate (Cayman Chemical, Ann Arbor, MI, USA). For steady-state kinetic analyses, varying concentrations of CDNB (0.005–2 mm) and PGH2 (0.08–40 μm for PGDS and 0.08–4 μm for PGES) were used while that of GSH was fixed at a saturating concentration (2 mm); substrate concentration ranges were empirically determined via pretesting. Kinetic parameters, including maximum reaction rate (Vmax), Michaelis constant (Km), and turnover number (Kcat), were calculated by nonlinear regression of hyperbolic plots using the SigmaPlot program with an enzyme kinetics module (ver. 12.5). All reactions were performed in triplicate, and values were expressed as the mean±SD.
Results
Identification of sigma- and mu-class GST genes/proteins in C. sinensis
The aa sequences of Clonorchis GST proteins retrieved from GenBank were compared with one another. After removing allelic and/or redundant entries with 95.0% identity cutoff, 5 sequences were finally selected to comprise the representative paralogs of sigma-class GST in C. sinensis. The redundancy was verified by checking their overlapped genomic loci. In GenBank, these proteins had been designated as GST (3 proteins) or prostaglandin-H2 D-isomerase (an alternative name of PGDS, 2 proteins). While keeping their original names, the identities of these proteins were distinguished by adding Arabic numerals as follows: CsGST-σ1 (AAD17488.1), CsGST-σ2 (ABA56496.1), CsGST-σ3 (ABC72085.1), CsPGDS1 (GAA52095.1), and CsPGDS2 (GAA54850.1). Two GST proteins (GAA30025.1 and GAA50904.1) that showed closer structural similarity toward mu-class GSTs were also identified (CGST-μ1 and CsGST-μ2, respectively).
Structural characterization
The primary sequences of the CsGST proteins were compared with those of sigma-class GSTs of other parasitic trematodes and those of human and rat HPGDSs. As shown in Fig. 1, the aa residues known as to constitute the GSH- and PGH2-binding site were tightly conserved in the primary structures of these proteins [12]. The characteristic secondary structure of GST proteins, which comprises 8 alpha helices and 4 beta sheets, was predictable in the CsGST proteins. However, the aa comprising the Ca2+-binding site in mammalian HPGDS [12] were not conserved in the trematode protein sequences. The tertiary structures of CsPGDS1 and CsGST-σ3 proteins were successfully simulated based on the F. hepatica sigma-class GST structure (PDB id. 2WB9-A) by using I-TASSER. The C-score, TM-score, and RMDS were 1.08, 0.86±0.07, and 3.3±2.3 Å for CsPGDS1 and 1.15, 0.87±0.07, and 3.2±2.3 Å for CsGST-σ3, respectively. The CsPGDS1 and CsGST-σ3 structures aligned well with those of human PGDS (O60760.3; 1.09, 0.86±0.07, and 3.2±2.2 Å) and CsGST-μ1 (0.56, 0.79±0.09, and 4.5±3.0 Å), which were similarly predicted with I-TASSER (Supplementary Fig. S1). The aa involved in the binding of GSH (green) or substrate (i.e., CDNB or PGH2; red) were positioned to occupy similar spaces in the aligned tertiary structures.
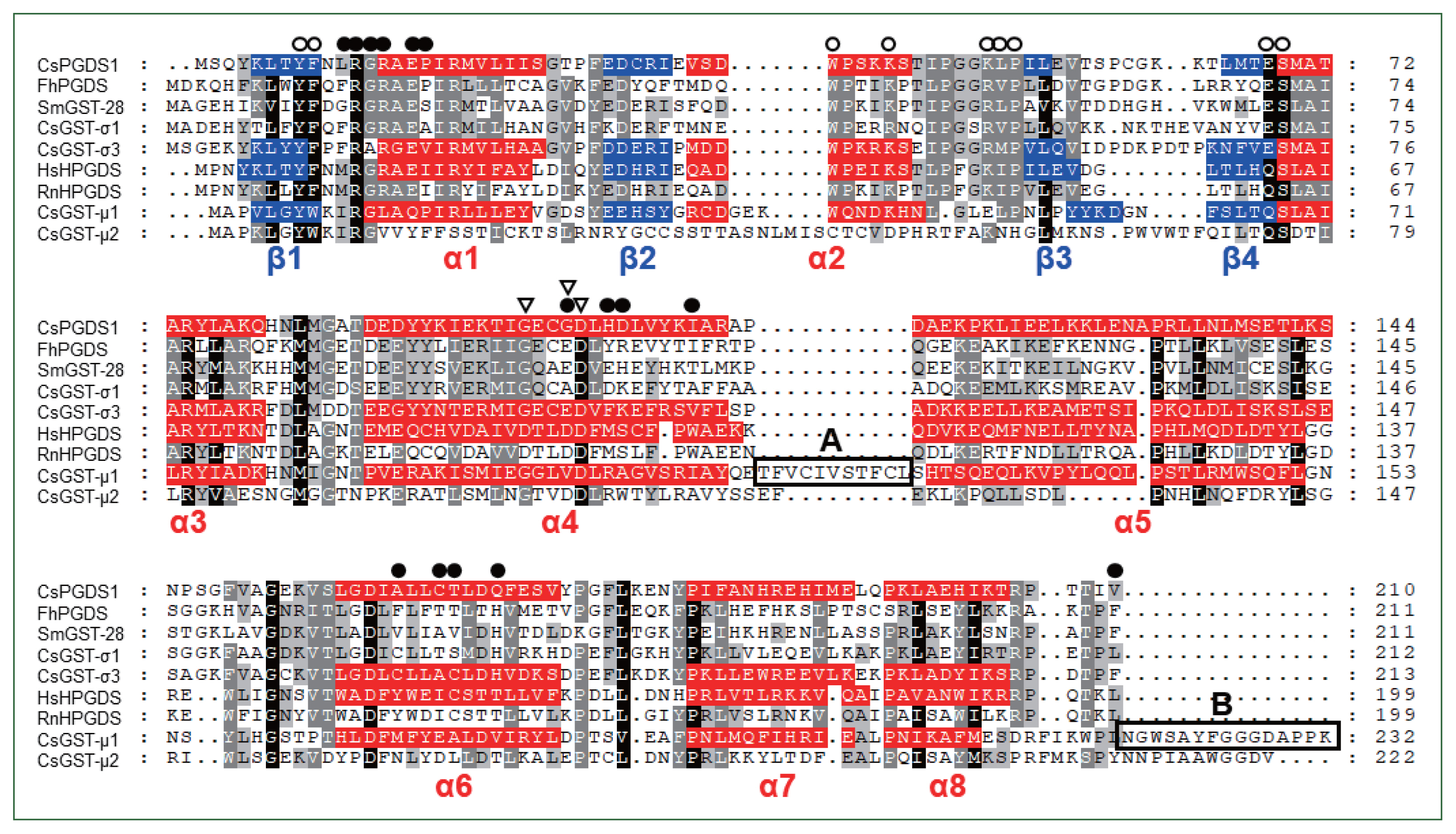
Comparison of primary structures between Clonorchis sinensis sigma-class GST and their trematode orthologs. Human and rat hematopoietic prostaglandin D synthase (HPGDS), as well as C. sinensis mu-class GSTs, were selected for the comparison. Dots in the alignment were introduced to increase identity values. Different shades of gray indicate the degrees of similarity among proteins. Secondary structural motifs predicted in the CsPGDS1 sequence are highlighted in red (alpha helix) or blue (beta sheet). Open circles, GSH-binding site; closed circles, PGH2-bindind site; open triangle, residues comprising the Ca2+-binding site in HsHPGDS. Boxes A and B marks aa stretches uniquely detected in CsGST-μ1. CsGST-μ1, C. sinensis (GAA30025.2); CsGST-μ2, C. sinensis (GAA50904.1); CsGST-σ1, C. sinensis (AAD17488.1); CsGST-σ3, C. sinensis (ABC72085.1); CsPGDS1, C. sinensis (GAA52095.1); FhPGDS, Fasciola hepatica (ABI79450.1); SmGST-28, Schistosoma mansoni (CCD60449.1); RnHPGDS, rat (NP_113832.1); HsHPGDS, human (O60760.3).
Phylogeny of trematode sigma-class GSTs
The number of sigma-class GST paralogs differed among parasitic trematodes. Multiple paralogous proteins were identified in the proteome databases of O. viverrini (5), P. westermani (3), and F. hepatica (2), whereas only a single orthologous protein was present in Schistosoma spp. A maximum likelihood tree demonstrated that the progenitor of the sigma-class GST gene had been duplicated in a common ancestor of trematode species (Fig. 2). One of the daughter copies was further multiplied during an early evolutionary period of the opisthorchiid lineage (closed arrowhead in Fig. 2), whereas the corresponding gene copy was deleted in the other trematode genomes. Another daughter copy (open arrowhead) was also multiplied in several trematode genomes such as P. westermani and Fasciola spp.
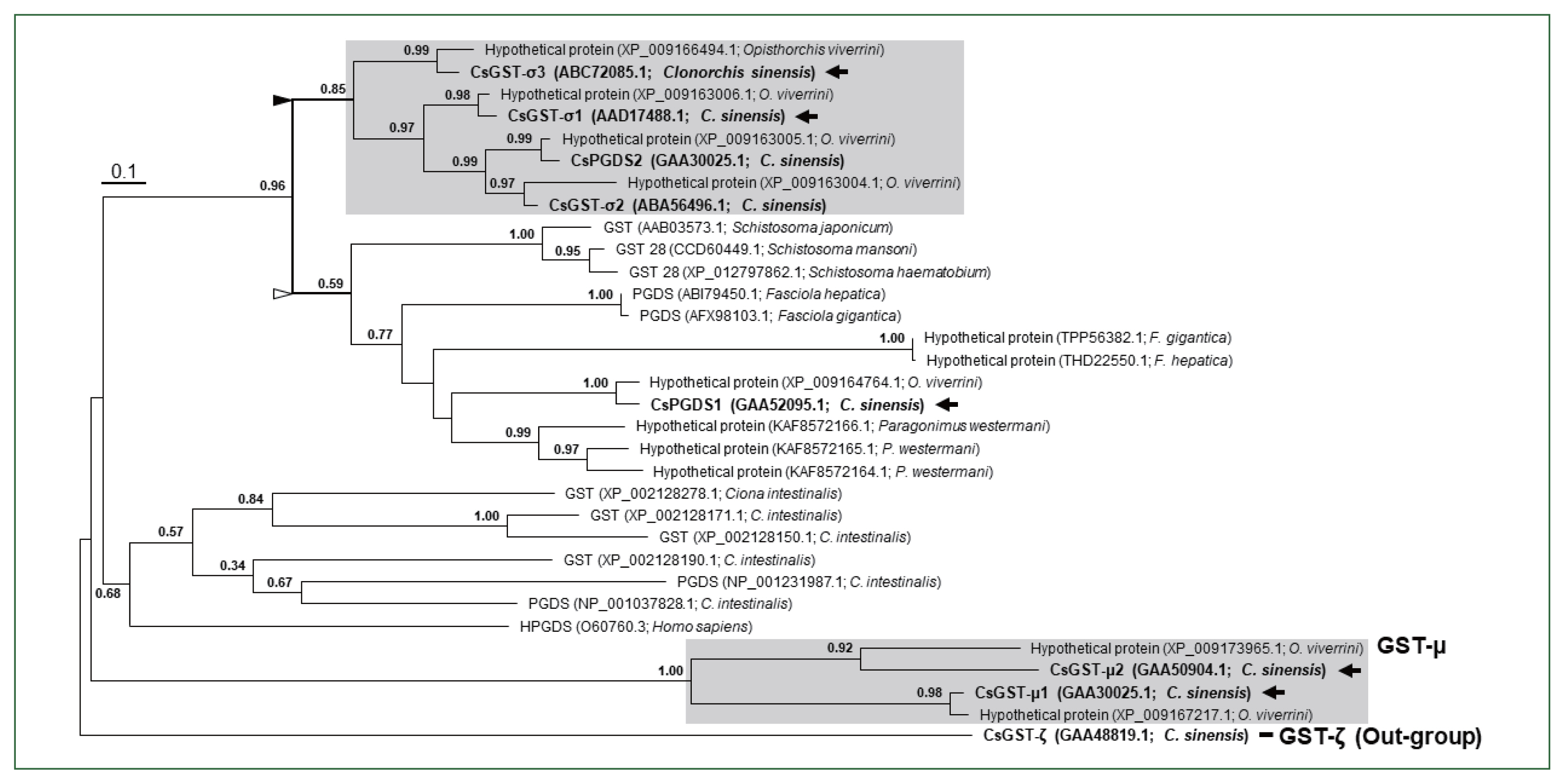
Phylogenetic analysis of Clonorchis sinensis sigma-class GSTs and their closest neighbors in trematode parasites. Mu-class GSTs identified in C. sinensis and Opisthorchis viverrini, and sigma-class GTSs/HPGDS in Ciona intestinalis and Homo sapiens were also included in the phylogenetic analysis. The maximum likelihood tree was rooted with a zeta-class GST of C. sinensis (GAA488191.1). Numerals at branching nodes are the Shimodaira–Hasegawa-like approximate likelihood ratios obtained with 1,000 replicas. Closed and open triangles mark each of the paralogous gene lineages generated by a gene duplication event in an ancestral trematode genome. Arrows indicate the Clonorchis proteins that were generated as recombinant proteins for examination of their enzymatic properties.
Enzymatic properties of recombinant CsGST proteins
The complete ORF sequences of CsGST genes were amplified from the C. sinensis cDNAs and expressed in E. coli to obtain recombinant proteins. During the preparations, 2 proteins were excluded because the ORF was corrupted by a premature stop codon (CsPGDS2) or because the recombinant form was expressed as a insoluble form (CsGST-σ2). Finally, 5 rCsGST proteins (rCsGST-σ1, rCsGST-σ3, rCsGST-μ1, rCsGST-μ2, and rCsPGDS1) were used for enzymatic analysis.
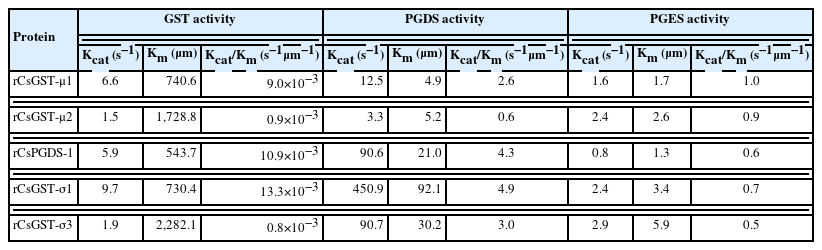
The specific GST activities of rCsGSTs against CDNB were 9.4±0.12, 0.2±0.09, 13.6±0.59, 9.6±0.02, and 1.3±0.15 μm product/ml/min/μg for rCsGST-μ1, rCsGST-μ2, rCsPGDS1, rCsGST-σ1, and rCsGST-σ3, respectively. The PGDS activities for these enzymes were 1.3±0.01, 0.8±0.07, 2.1±0.12, 1.8±0.01, and 0.9±0.02 μm product/ml/min/μg, respectively. These enzymes were also involved in the conversion of PGH2 to PGE2, although the specific PGES activities were slightly lower than those for PGD2 synthesis (0.7±0.03, 0.5±0.01, 0.4±0.01, 0.5±0.01, and 0.3±0.00 μm product/ml/min/μg for rCsGST-μ1, rCsGST-μ2, rCsPGDS1, rCsGST-σ1, and rCsGST-σ3, respectively) (Fig. 3).
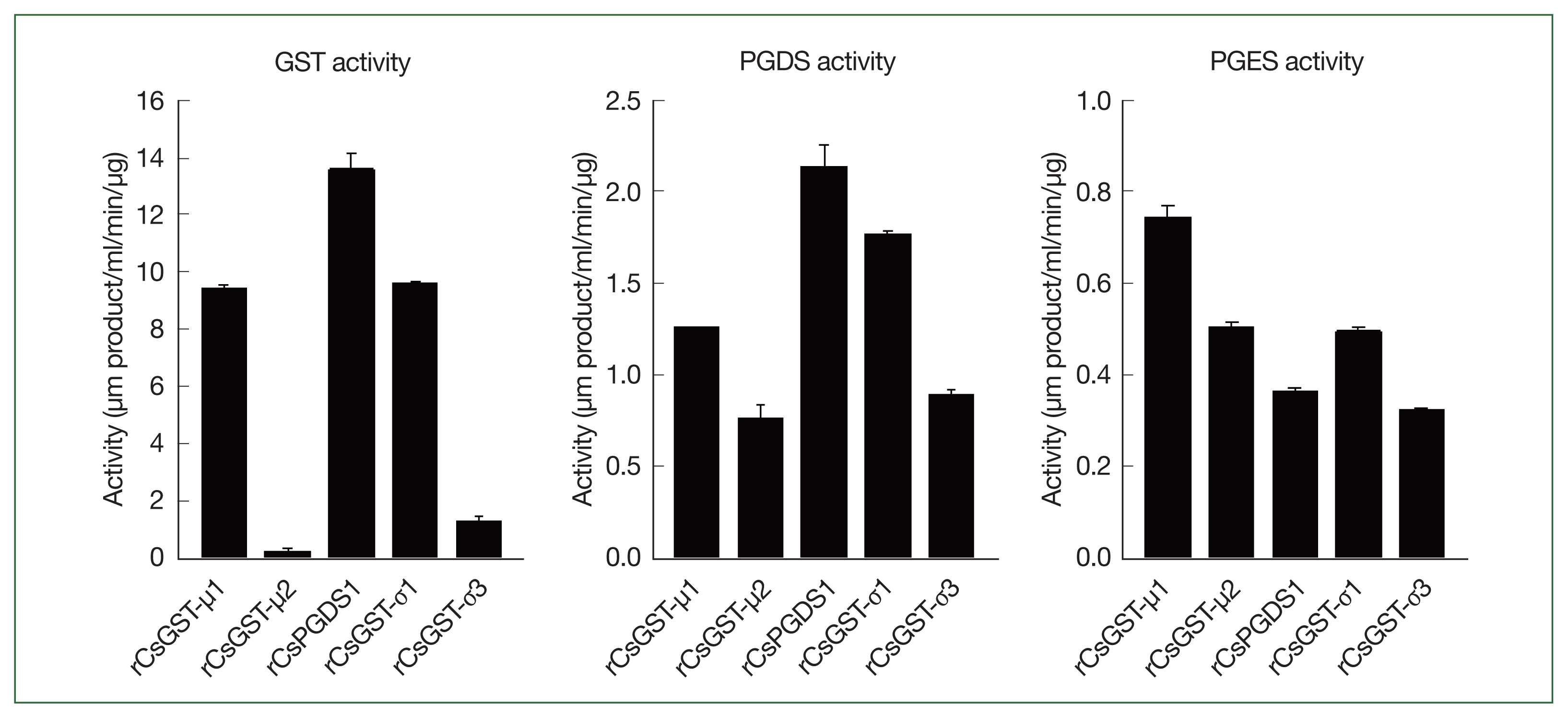
Glutathione transferase and prostaglandin synthase activities of Clonorchis sinensis recombinant GST proteins. Enzyme activities were determined by using CDNB (GST activity) or PGH2 (PGDS and PGES activities) as well as reduced glutathione. Measurements were performed in triplicate, and the results are expressed as mean±SD.
To compare the substrate-binding affinity and enzymatic efficacy among the rCsGST proteins, a series of steady-state kinetic parameters were determined for the generation of GS-DNB conjugate, PGD2, or PGH2 by using their respective substrates. The Vmax and Km values calculated by the kinetic analyses were substantially different among these rCsGST proteins (Fig. 4). More importantly, these proteins all exhibited much greater affinity toward PGH2 than toward CDNB. The Kcat/Km values, which is a measure of the catalytic efficiency of an enzyme, were also considerably increased when these proteins catalyzed the production of PGD2 or PGE2 (0.6–4.9 and 0.5–1.0 s−1μm−1, respectively); the Kcat/Km values ranged from 0.8×10−3 to 13.3×10−3 s−1μm−1 for the formation of the GS-DNB conjugate (Table 1).
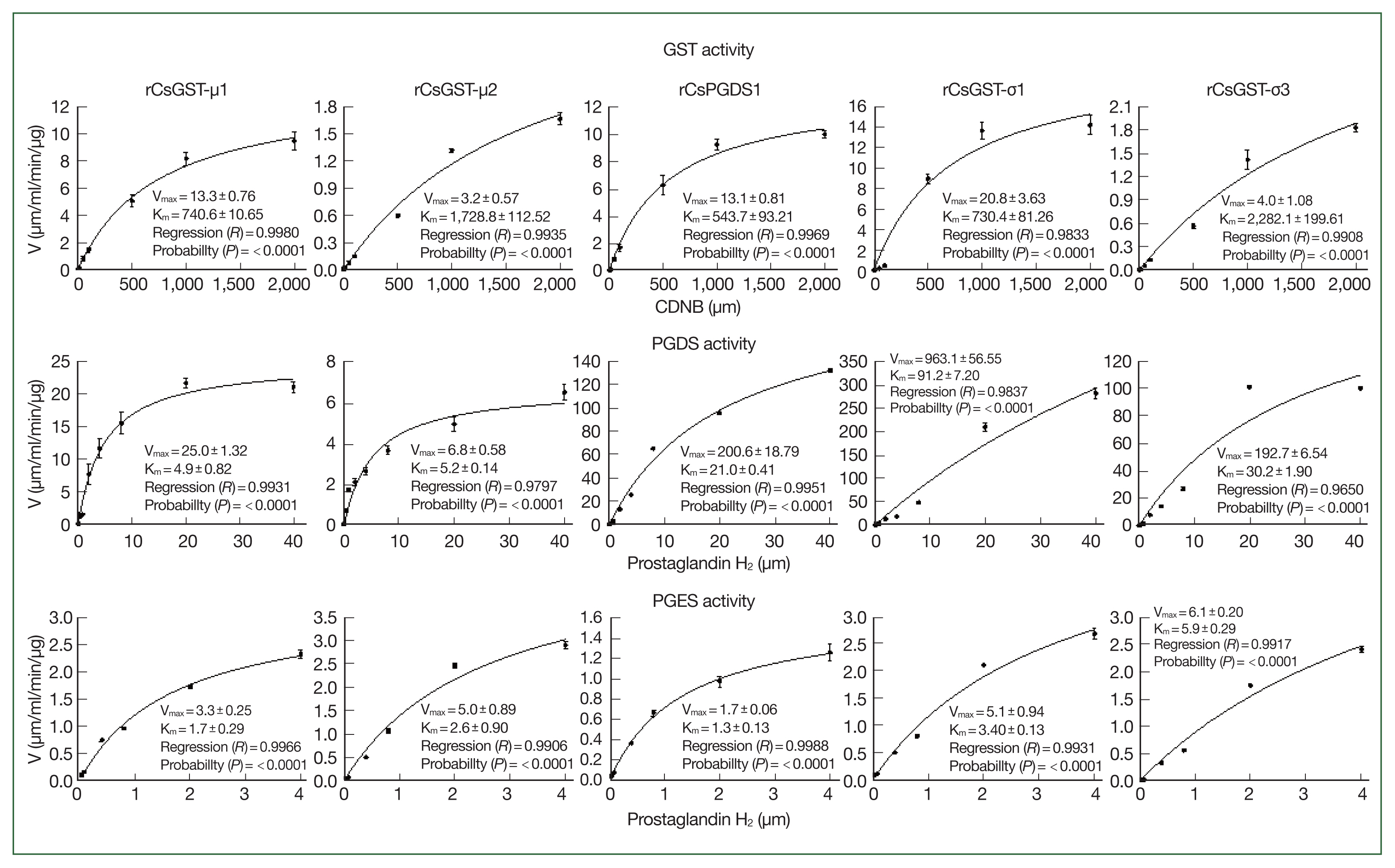
Steady-state kinetic analyses of Clonorchis sinensis recombinant GST proteins (rCsGSTs) for their glutathione transferase and prostaglandin synthase (PGDS and PGES) activities. Kinetic analyses were conducted by varying the concentrations of specific substrates as indicated in the X-axis tick marks while that of GSH was fixed at a saturating concentration. The kinetic parameters, Vmax and Km, were calculated by the nonlinear regression of hyperbolic plots (curves in each of the graphs) using the SigmaPlot program with an enzyme kinetics module (ver. 12.5). Measurements were performed in triplicate, and results are expressed as mean±SD.
Discussion
This study comprehensively screened sigma-class GST proteins present in the proteome of C. sinensis. The proteins shared structural properties with HPGDs identified in mammalian vertebrates, including human. The phylogenetic analysis based on the conservation patterns of primary protein structures demonstrated that gene(s) encoding the sigma-class GST proteins had undergone gene duplication events that specifically occurred in an opisthorchiid genome ancestral to C. sinensis, O. viverrini, and several other trematode lineages. Recombinant forms of the sigma-class GSTs, as well as mu-class proteins, exhibited considerable GST and PGS activities. However, the relative binding affinities toward PGH2 (average Km of 30.7 and 3.0 μm for the PGDS and PGES activities, respectively) were much greater than those toward CDNB (average Km of 1,205.1 μm). Comparison of the enzymatic efficiency suggested that the C. sinensis GST proteins function more effectively as a PGS (average Kcat/Km of 3.1 and 0.7 s−1μm−1 for PGDS and PGES, respectively) than as a GST (average Kcat/Km of 7.0×10−3 s−1μm−1).
Genetic dosages responsible for the expression of sigma-class GST varied among trematode species (Fig. 2). Five paralogous genes were found in the C. sinensis and O. viverrini genomes, whereas 2 and 3 were found in the genomes of Fasciola spp. and P. westermani, respectively. However, only a single homologous gene was identified in each of the schistosome genomes. The phylogenetic analysis based on their encoded aa sequences suggested that the copy number variation resulted from genetic duplication and/or following deletion events that had specifically occurred in the trematode species/lineages (Fig. 2). A difference in the copy number has also been observed with the mu-class GST genes, where the copy numbers were greater in Fasciola spp. than in opisthorchiids [8,26]. Considering that the copy number of functional genes has a profound effect on their phenotypic expression [27], the differential copy numbers of GST genes would reflect the specifically evolved roles in the given trematode species. Clonorchis sinensis and F. hepatica secrete sigma- and mu-class GST proteins into surrounding environments. However, the relative amounts of these GSTs differ between these species; the sigma-class proteins, especially CsGST-σ3, are prominently found in the C. sinensis ESP [9], whereas the mu-class members are more abundant in that of F. hepatica [17,21]. The major component of Opisthorchis felineus ESP is also a sigma-class GST protein [28]. Furthermore, inductive expression profiles either during development or against exogenous stimuli substantially differ among the GST genes in C. sinensis [26]. Thus, the GST proteins, which have previously been known to function as phase II antioxidant enzymes in intracellular spaces, are presumed to have gained additional roles involved in the host-parasite interplays in these liver flukes. Despite the similar tissue tropism toward the biliary tracts of mammalian hosts, C. sinensis/O. viverrini and Fasciola spp. show differences in the routes and periods of penetration of the biliary lumen [29,30]. Factors derived from the differential host environments may have provided a selection pressure for the functional diversification of these trematode GST proteins.
Helminths parasitizing mammalian hosts might have evolved molecular machineries associated with regulating host immune responses and sequestering harmful endogenous/exogenous oxidants. Prostaglandins generated by these tissue-invading helminth parasites are essential for establishing and maintaining infection in their definitive hosts. In the extracellular host spaces, the eicosanoids are intimately involved in modulating host immune responses [19,31]. During chronic infection, these molecules induce pathophysiological changes such as pyrexia, alteration of cellular metabolism, and neoplasia in the affected tissues or hosts [32]. Meanwhile, PGs produced within the parasite seem to play key roles in association with the parasite penetration of host skin [33] and embryogenesis [17]. Considering the spatial expression patterns [9] and enzymatic properties (Table 1), the CsGST-μ and CsGST-σ proteins can provide dual activities for the immunomodulation and antioxidant defense in C. sinensis. Whether the GST proteins directly participate in de novo synthesis of PGs from arachidonic acids in the trematode cells is currently unclear because the parasites do not encode the cyclooxygenase isozymes responsible for the generation of PGH2 [14]. The C. sinensis ESP stimulates the expression of host genes involved in the generation of immunomodulatory eicosanoids [34] and the protein components penetrate the plasma membranes of surrounding host cells [35]. Therefore, the C. sinensis GSTs in ESP are likely to cooperate with host enzymes to generate PGD2 and/or PGE2 from PGH2 in intracellular spaces. Inflammatory responses accompanying the infiltration and activation of immune cells [24] may provide an opportunity to establish the intracellular PG-generating environments.
Human HPGDS (single sigma-class GST member in mammals) and GSTMs (mu-class GST members) are cytosolic proteins that also exhibit the PGDS and PGES activities, respectively [12,36]. The CsGST-σ proteins share structural and enzymatic properties with the human HPGDS (Figs. 1, 3). Interestingly, the Clonorchis proteins could also catalyze conversion of PGH2 to PGE2, although their specific PGES activities and catalytic efficiencies were much lower than those for PGES (Fig. 3; Table 1). The CsGST-μ proteins had enzymatic properties similar to those of the CsGST-σ proteins. The highest expression of human HPGDS mRNA is found in macrophages [5], which are highly activated during C. sinensis infection [24]. Therefore, the eicosanoid molecules may play important roles in immune responses invoked by C. sinensis such as during type 2 inflammation and fibrotic responses [24]. The human HPGDS contains 3 conserved Asp residues forming Ca2+ ion-binding sites in the primary structure (Fig. 1). The cytosolic influx of Ca2+ ion is commonly observed in activated immune cells, and the interaction between dimeric HPGDS and Ca2+ ion increases the PGDS activity up to 150% of the basal level in a concentration-dependent manner [12]. The invariant Asp residues were not detected in the corresponding regions of C. sinensis proteins (Fig. 1). Further investigations on the inductive expressions of PG-related genes in affected host cells may help generate insight into the roles of PG molecules in C. sinensis infection.
In summary, we showed that the sigma- and mu-class GSTs expressed in C. sinensis have variable PGDS and PGES activities in addition to the conventional GSH-conjugating activity. These proteins displayed much greater affinities toward PGH2 than those toward CDNB. The efficiency of PGS activities, expressed as Kcat/Km obtained by steady-state kinetic analyses, was also higher than that of the GST activity. Therefore, C. sinensis GST proteins may act as antioxidant enzymes in parasitic parenchyma, where the concentration of the proteins and probable substrates are relatively high. Conversely, these proteins are probably responsible for the generation of PGs such as PGD2 and PGE2, after being secreted into the surrounding host environments. The antioxidant and immunoregulatory roles are pivotal for the survival of the parasite and maintenance of the infection in definitive hosts. Therefore, these GST proteins can provide effective targets during investigations of therapeutics or vaccines to control clonorchiasis that is highly prevalent in Asian countries such as Korea.
Supplementary Information
Acknowledgments
This work was supported by the Basic Science Research Programs of the National Research Foundation of Korea (NRF), funded by the Ministry of Education (NRF-2017R1D1A1B03028355) and by the Gachon University research fund of 2018 (GCU-2018-0329) to YAB.
Notes
The authors declare no conflict of interest related to this study.
Conceptualization: Kim J, Sohn WM, Bae YA
Data curation: Kim J, Bae YA
Formal analysis: Kim J
Funding acquisition: Bae YA
Investigation: Kim J, Bae YA
Resources: Sohn WM
Supervision: Bae YA
Validation: Sohn WM, Bae YA
Visualization: Kim J, Bae YA
Writing – original draft: Bae YA
Writing – review & editing: Bae YA