Prevalence of asymptomatic malaria in high- and low-transmission areas of Tanzania: The role of asymptomatic carriers in malaria persistence and the need for targeted surveillance and control efforts
Article information
Abstract
As many countries implement different programs aimed at eliminating malaria, attention should be given to asymptomatic carriers that may interrupt the progress. This was a community-based cross-sectional study conducted in Tanzania from December 2022 to July 2023 within 4 villages from each of the 3 regions, Geita and Kigoma, which are high malaria transmission, and Arusha, which is low transmission. Malaria was diagnosed in asymptomatic individuals aged 1 year and older using the malaria rapid diagnostic test and light microscope. A total of 2,365 of 3,489 (67.9%) participants were enrolled from high-transmission villages. The overall prevalence was 25.5% and 15.8% by malaria rapid diagnostic test and light microscope, respectively. Using the respective tools, the prevalence was significantly higher at 35.6% (confidence interval (CI)=23.6–49.9) and 23.1% (CI=16.2–35.1) in the high-transmission regions (Geita and Kigoma) compared with 2.9% (CI=1.1–3.5) and 1.1% (CI=0.7–1.8) in the low-transmission region (Arusha). Children younger than 15 years and males accounted for the greatest proportion of infections. In the study area, the prevalence of asymptomatic cases was higher than that of reported symptomatic cases in health facilities. We hypothesize that these parasite reservoirs may contribute to the persistence of malaria in the country. Therefore, to achieve comprehensive malaria control in the country, the surveillance and screening of asymptomatic malaria cases are vital.
Malaria is a life-threatening disease caused by the Plasmodium parasite and is endemic in 85 countries. In 2022, 249 million malaria cases and 608,000 malaria deaths were reported globally, with the World Health Organization (WHO) African regions disproportionally sharing 94% of cases and 95% of deaths [1]. Tanzania, a country in sub-Saharan Africa (SSA), is among the 10 countries with the highest malaria cases and deaths. In 2021, Tanzania accounted for 3.1% of the global malaria cases and 4.1% of global malaria deaths. In East and Southern Africa, Tanzania contributed to 12.8% of the regional cases of malaria [2].
The Government of Tanzania, through the National Malaria Control Program and in collaboration with partners, has made significant achievements in the fight against malaria, resulting in a remarkable reduction in the burden of the disease. According to the National Malaria Indicator Survey, the prevalence of malaria in children younger than 5 years dropped from 14.8% in 2015 to 7.5% in 2017. Malaria cases declined from 7.7 million cases in 2015 to 5.9 million in 2020. Likewise, the incidence of malaria per 1,000 of the population dropped from 162 in 2015 to 106 in 2020 [3]. Despite these significant milestones, Tanzania remains among the 19 SSA countries, together with India, that carry 85% of the global malaria burden [1]. In alignment with the WHO’s goal of achieving a 90% reduction in the malaria burden by 2030 [4], monitoring asymptomatic malaria is critical, as we believe it plays a significant role as a silent reservoir for the prevalence of symptomatic malaria.
Asymptomatic malaria infections are very prominent in countries in SSA [5]. Despite its significance in sustaining malaria transmission within the population, most National Malaria Control Programs have underestimated and overlooked asymptomatic malaria. Although previous studies have shown high heterogeneity of malaria transmission in Tanzania [6,7], information on the prevalence of asymptomatic malaria at the microgeographic level is limited [6]. Given the substantial evidence that asymptomatic malaria plays a significant role in the transmission of infections [8,9], monitoring and surveilling these cases are crucial for controlling the spread of malaria.
In this community-based cross-sectional study, conducted from December 2022 to July 2023 in 12 villages in Tanzania, we assessed the prevalence of malaria infections in the population of asymptomatic individuals aged 1 year and older. The study was planned and conducted in accordance with the protocol approved by the National Institute for Medical Research, a division of the Ministry of Health in Tanzania (approval No. NIMR/HQ/R.8a/Vol. IX/4114), as well as the Ethical Review Board at Kangwon National University (approval No. KWNUIRB-2022-06-008). The study was conducted in accordance with the relevant guidelines and regulations. All participants provided informed consent at the time of enrollment.
Eight of the studied villages were located in areas with high malaria transmission, and 4 villages were in areas with low transmission. Simply, we purposively selected 2 regions (Kigoma and Geita) within the area of high malaria transmission and selected Arusha from within the low-transmission area (Fig. 1A) [10]. We randomly selected 2 districts from each region, followed by randomly selecting 2 villages from each district. The districts and respective villages included the Geita region (Nyang’hwale district: Nyangalamila and Kayenze; Chato district: Ihanga and Rwantaba), the Kigoma region (Kibondo district: Kumuhasha and Bunyambo; Kasulu district: Nyamnyusi and Mugombe), and the Arusha region (Meru district: Maji ya chai and Ngurudoto; Arusha district: Bwawani and Themi ya simba) (Table 1). This is one of the few studies that have assessed the prevalence of malaria in asymptomatic individuals across all age groups as well as in different malaria transmission hotspots in Tanzania. The expected total number of participants was n=3,360, distributed across 12 villages (n=280). The sample size was calculated using a proportional formula, considering a malaria prevalence of 11.3% per 1,000 [10] population in Tanzania, a margin of error of 0.01, a standard normal deviation of 1.96 at a 95% confidence interval (CI), and a 10% nonresponse rate. This study provides valuable data for policy makers, decision-makers, and program planners to better understand the burden of malaria and implement effective interventions aimed at reducing its public health impact.
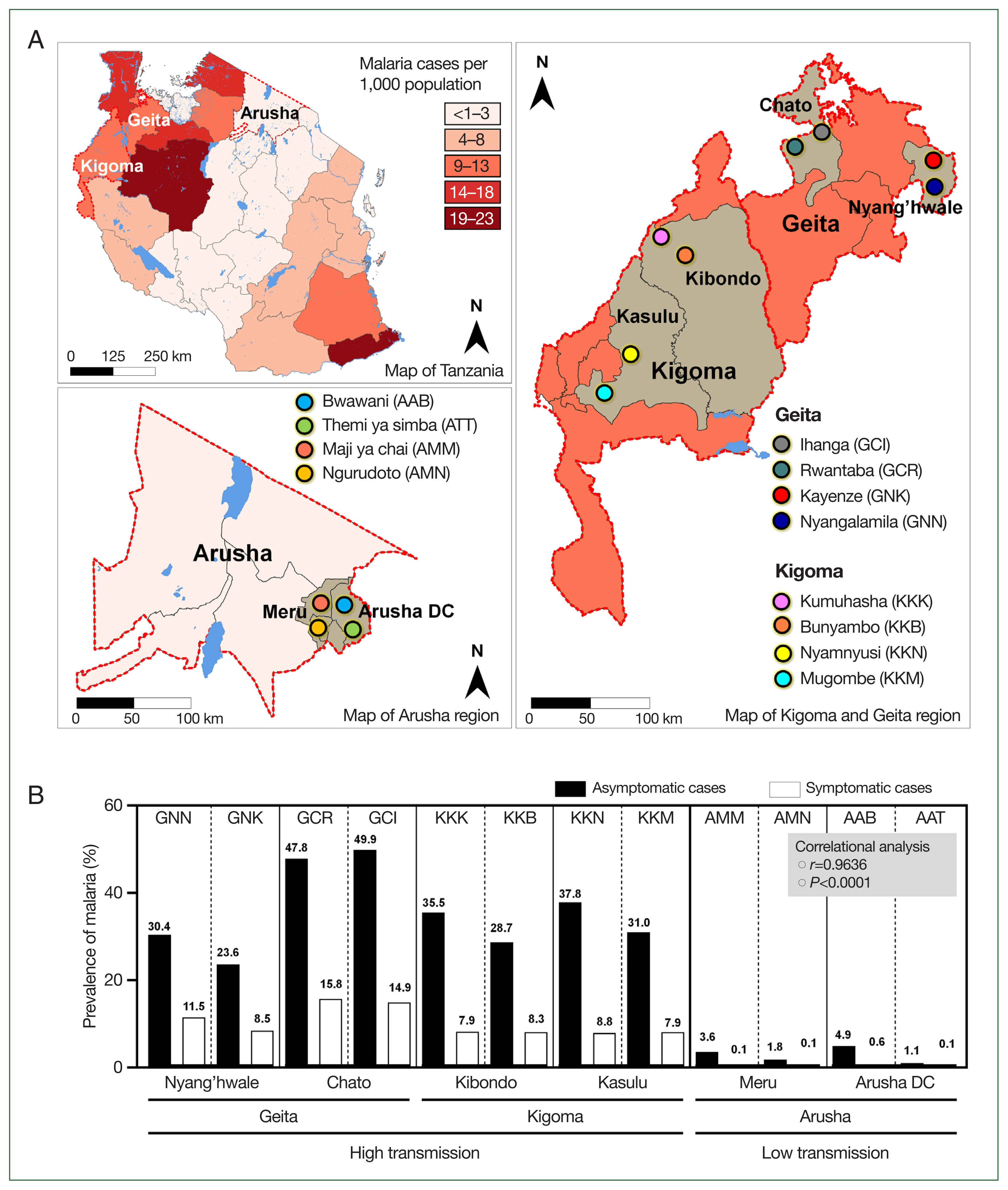
Tanzania map. (A) Tanzania map showing regions (Geita and Kigoma for high transmission setting, and Arusha from low transmission setting) and districts (Geita: Chato and Nyang’hwale, Kigoma: Kasulu and Kibondo, and Arusha: Arusha DC and Meru) that were involved in the study. Patterns of color indicate the distribution and prevalence of malaria cases in 2022 as were reported by the President Malaria Report, 2023. (B) Prevalence of asymptomatic malaria from studied villages and prevalence of symptomatic malaria cases from health facilities within respective villages as recorded from the District Health Information System 2 from December 2022 to July 2023, a period when the study was conducted. Asymptomatic malaria cases from villages positively correlated with symptomatic malaria cases reported in village health facilities by correlational analysis.
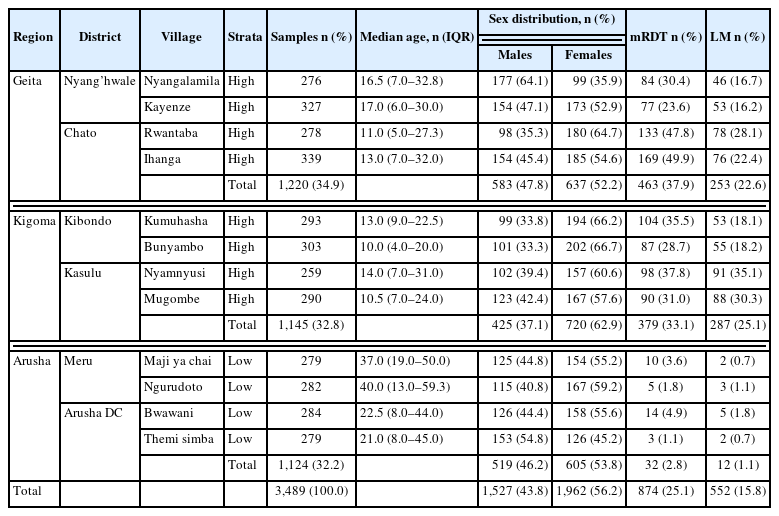
Distribution of samples, age, sex of participants and asymptomatic malaria results within studied sites by mRDT and LM
With the guidance of the village chairpersons and community health workers, a team of researchers visited the participant houses. At each household, a written informed consent/assent was obtained from the participants or the guardians/parents of the participant in the case of children (age less than 18 years). Through trained personnel, pretested structured questionnaires were used to collect the participants’ sociodemographic information. A medical doctor examined the clinical manifestations of malaria to identify participants without malaria symptoms. Thereafter, 4 to 5 consented participants without symptoms were randomly selected per each household. If there were fewer than 4 members in the household, all were selected. Venous blood was collected from each participant into heparinized tubes (BD Vacutainer plastic heparin tubes; BD, Franklin Lakes, NJ, USA), and malaria was diagnosed according to the manufacturer’s guidelines using histidine-based rapid kits (Bioline Malaria Ag P.f/Pan; Abbott, Chicago, IL, USA). Blood samples were then transported to a nearby health facility, and trained microscopists prepared and microscopically examined thin blood Giemsa-stained slides for malaria infections. At least 20 fields on each slide were examined using a light microscope (LM) with a 100×objective lens. The LM diagnosis was conducted in accordance with the WHO Malaria Microscopy Standard Operating Procedure. All participants who tested positive for malaria by malaria rapid diagnostic test (mRDT) were treated with the first-line antimalarial for uncomplicated malaria in Tanzania, artemether–lumefantrine, in accordance with the country’s malaria treatment guidelines.
Overall, 3,489 participants from the 12 villages met the enrollment selection criteria and were tested for malaria infections by both mRDT and LM (Table 1). Of the participants, 1,527 (43.8%) were male, whereas 1,962 (56.2%) were female. A total of 2,365/3,489 (67.8%) individuals were from the 8 villages within the high-malaria-transmission settings, Geita (n=1,220; 51.6%) and Kigoma (1,145; 48.4%), whereas 1,124/3,489 (32.2%) were from the 4 villages in Arusha within the low-transmission area. The mean (±SD) age of the participants was 19.6±19.2 years, and the median age was 13 (interquartile range, 6–28) years.
From the high-transmission settings (Geita and Kigoma, n=2,365), the prevalence of malaria was 35.6% (n=842; CI=23.6–49.9) and 23.1% (n=540; CI=16.2–35.1) as compared with the low-transmission region (Arusha, n=1,124), where the prevalence was 2.8% (n=32; CI=1.1–3.6) and 1.1% (n=12; CI=0.7–1.8) by mRDT and LM respectively. In the comparison of the prevalence by regions, a nonsignificant difference was revealed between Geita and Kigoma by both mRDT and LM (P>0.05); however, the infections in the 2 regions were significantly higher than those in Arusha using the respective tools (P<0.001). Overall, the prevalence detected by mRDT was 874 (25.1%), significantly higher than that identified by LM, which was 552 (15.8%) (P=0.0075). This observation may be a result of the reduced sensitivity of LM at low parasite densities in asymptomatic infections [11] or the persistence of malaria antigens in the blood circulation even after parasite clearance, leading to false-positive results in mRDT [12] (Table 1).
The prevalence of asymptomatic malaria was disproportionately distributed in the studied villages (P=0.0068). Comparatively, by mRDT, the highest proportion of infections occurred in Ihanga village (49.9%), followed by Rwantaba village (47.8%), both of which were from Chato district in the Geita region. Furthermore, Nyamnyusi village (37.8%) and Kumuhasha village (35.5%), located in the Kasulu and Kibondo districts, respectively, were the third and 4th villages with the highest infection rates. Of the districts, asymptomatic malaria was higher in Chato (48.9%), followed by Kasulu (34.4%), Kibondo (32.1%), and Nyang’hwale (27.0%). On the other hand, Arusha district (in the low-transmission area) had a higher prevalence (3.0%) than Meru (2.7%) did. The difference in infections was not significant between districts from high-transmission (P=0.6521) and low-transmission (P=0.8089) settings. In all cases, the difference of malaria infections in the 2 villages from a similar district was not significant (P>0.05), indicating similarities of asymptomatic infections in microgeographic areas that were in close location (Table 1). According to data from the District Health Information System 2, there was a significant positive correlation between the prevalence of asymptomatic malaria in the general population and the number of malaria cases reported at the village health facilities during the same study period (P=0.0001, r=0.9636) (Fig. 1B). This strong correlation suggests a close link between the prevalence of asymptomatic malaria in the broader community and the number of symptomatic malaria cases reported in local health facilities. This observation underscores the importance of further investigation to understand the dynamics of transmission, particularly the role of asymptomatic individuals in sustaining the prevalence of malaria in the population.
In both settings, the prevalence of asymptomatic malaria was significantly elevated in older children (age 5–15 years; 490 of 874 (56.1%)), followed by children younger than 5 years (166 (19.0%); P=0.0001). Thereafter, the prevalence significantly decreased as the participants’ age increases (Fig. 2A; Supplementary Table S1). This association of infections with age can be attributed to the intense transmission of malaria, especially in areas with high transmission, which elevates cases into the active reservoirs. School-aged children (5–15 years) are known to have considerably low preventive measures against malaria transmission [13,14], which might contribute to the higher prevalence in this group. Moreover, the prevalence of asymptomatic infections is known to be inversely related to the development of partial immunity, which is reported to be age dependent [15]. The observed trend of asymptomatic malaria was similar to previous reports in symptomatic cases in Kyerwa (a high-transmission area) [14], which highlights the role played by age groups in both symptomatic and asymptomatic malaria in Tanzania.
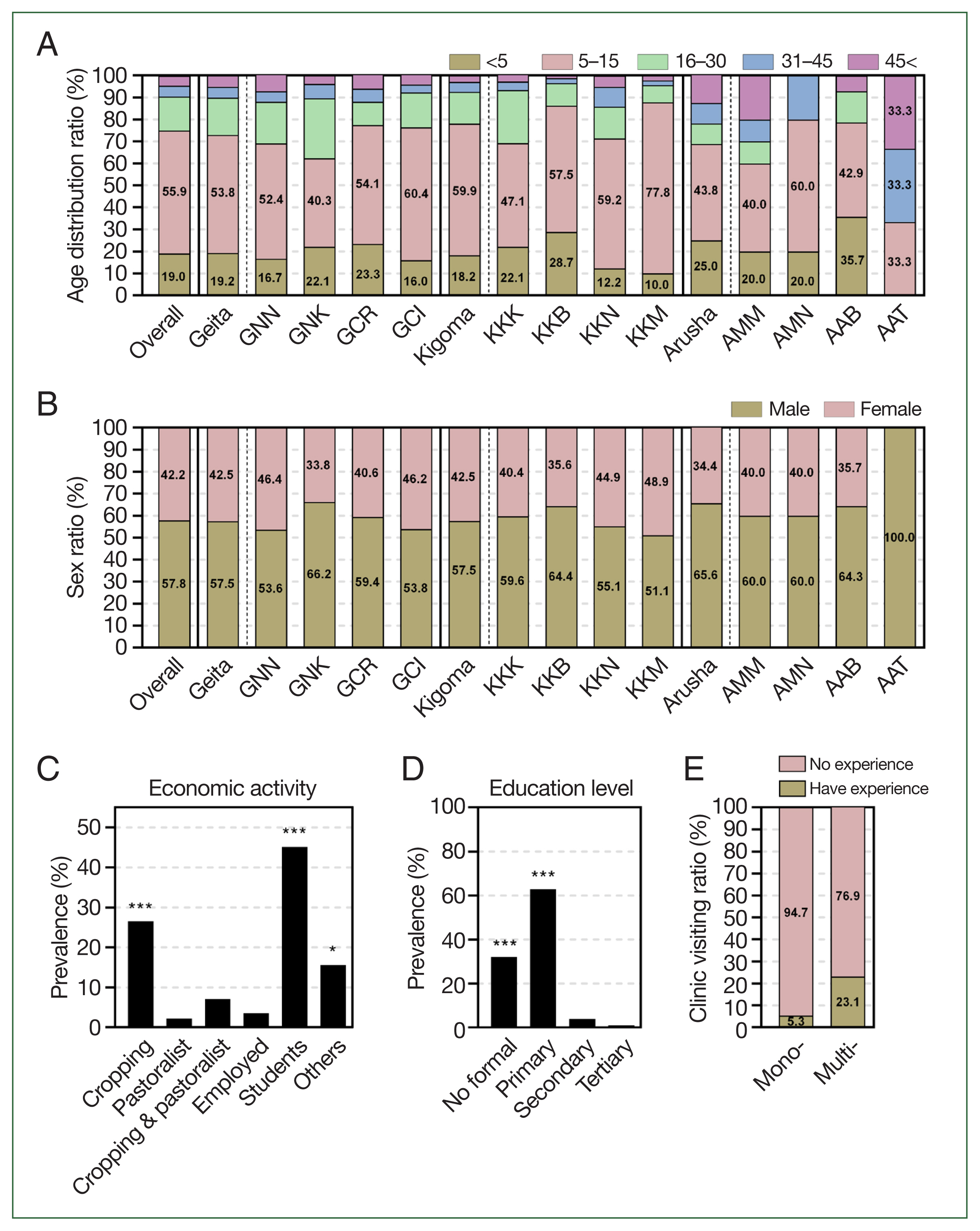
Prevalence of asymptomatic malaria by demographic information. (A) By age, overall prevalence was significantly higher in school aged children (5–15 years) than other age groups by one-way ANOVA (P=0.0001), followed by young children (<5 years) then prevalence significantly decreased by the increase in age of participants. (B) By sex, prevalence of asymptomatic malaria was significantly higher in males than females in both settings by paired sampled t-test (P<0.0001). (C) By economic activities, prevalence of asymptomatic malaria was significantly higher students and crop cultivating participants. Statistical significance was indicated by *P<0.05, **P<0.01, and ***P<0.001 using one-way ANOVA analysis. (D) By education level, prevalence of asymptomatic malaria was significantly high to participants without formal education and those with primary education, then gradually decreased with the increase in education level to tertiary education. Statistical significance was indicated by *P<0.05, **P<0.01, and ***P<0.001 using one-way ANOVA analysis. (E) By experience of clinic visiting, the experience of participants visiting health facilities for malaria symptoms was higher in asymptomatic cases with co-infections (Multi-) than those with mono-infections (Mono-). GNN, Nyangalamila; GNK, Kayenze; GCR, Rwantaba; GCI, Ihanga; KKK, Kumuhasha; KKB, Bunyambo; KKN, Nyamnyusi; KKM, Mugombe; AMM, Maji ya chai; AMN, Ngurudoto; AAB, Bwawani; AAT, Themi ya simba.
In addition, based on the mRDT results, asymptomatic malaria infections were significantly more common in males (505 of 874, 57.8%) compared with females (369 of 874, 42.2%) (P=0.0022) (Fig. 2B; Supplementary Table S2). With regard to economic activities, asymptomatic malaria infections were significantly more prevalent among farmers (232 cases, 26.5%) and students (394 cases, 45.1%) (P<0.0001) (Fig. 2C; Supplementary Table S2). Most students were from crop-cultivating families. Moreover, participants without formal education (280 cases, 32.0%) and those with primary education (550 cases, 62.9%) had a significantly higher prevalence of asymptomatic malaria (P<0.0001) compared with those with secondary education (35 cases, 4.0%) and tertiary education (9 cases, 1.0%) (Fig. 2D; Supplementary Table S1). These observations are further supported by previous studies, which suggest that variations in the incidence of malaria within populations may be influenced by factors such as gender roles, behavioral practices, sleeping patterns, hormonal differences, and genetic factors of the host. These determinants play a significant role in shaping individual susceptibility to malaria and contribute to disparities in the infection rates across different demographic groups [16]. In rural areas, men tend to stay outdoors until late at night. Likewise, farming and fishing activities mostly involve men. Moreover, it has been suggested that men have lower immune responses against diseases than women do [17]. It is crucial to understand how various demographic factors influence malaria transmission. This study emphasizes the importance of expanding malaria control and surveillance programs in Tanzania to include asymptomatic populations with special attention paid to the effect of different demographic patterns.
Using Malaria Ag P.f/Pan RDTs, of the 842 samples collected from high-malaria-transmission settings, 128 (15.2%) tested positive for Pan/Pf co-infections, whereas 6 of 32 samples (18.8%) from low-transmission settings also tested positive for the same. In addition, mono-infections of non–Plasmodium falciparum species (Pan positive) were observed in 9 (1.1%) participants exclusively from high-transmission villages. Mono-infections of P. falciparum were detected in 705 samples (83.7%) from high-transmission villages and 26 samples (81.2%) from low-transmission villages. These findings correlate with those of a study of 100 health facilities across 10 regions, which observed that symptomatic cases of P. falciparum were prevalent in all studied areas, whereas Plasmodium ovale and Plasmodium malariae covered only some regions [18]. Further analysis of premedical history revealed that 39 of 740 participants (5.3%) with mono-infections of either P. falciparum or Pan parasites and 31 of 134 participants (23.1%) with co-infections of both P. falciparum and Pan species reported prior visits to health facilities for malaria symptoms (Fig. 2E). This observation is supported by previous studies reporting that individuals who are co-infected have a higher chance of developing symptoms than those with mono-infections do [19].
To accelerate the progress toward the elimination of malaria in Tanzania, we recommend intensifying control strategies to include asymptomatic carriers, as they may hinder efforts to eliminate the disease. Although individuals with asymptomatic infections do not display symptoms, they contribute substantially to the prevalence of symptomatic malaria and are often associated with high levels of gametocytes, thus posing a challenge to elimination efforts [20]. This study provides baseline data for policy makers and malaria control stakeholders in Tanzania. We hypothesize that these parasite reservoirs may contribute to the persistence of malaria in both low- and high-transmission areas within the country. Therefore, to eliminate malaria, targeted interventions for this group are crucial.
Notes
Author contributions
Conceptualization: Mazigo E, Manjurano A, Han JH
Data curation: Mazigo E, Jun H, Louis JM, Muh F, Cha SH, Lee SJ, Na S, Geodfrey S, Han ET, Todd J, Manjurano A, Han JH
Formal analysis: Mazigo E, Jun H, Lee WJ, Louis JM, Fitriana F, Syahada JH, Muh F, Ahmed MA, Kija N, Geodfrey S, Han JH
Funding acquisition: Han JH
Investigation: Mazigo E, Jun H, Lee WJ, Lee SJ, Na S, Han ET, Todd J, Manjurano A, Han JH
Methodology: Mazigo E, Han JH
Project administration: Han JH
Resources: Mazigo E, Han JH, Kija N, Geodfrey S, Todd J, Manjurano A
Software: Mazigo E, Cha SH, Chun W, Park WS, Kija N, Geodfrey S
Supervision: Manjurano A, Han JH
Validation: Jun H, Lee WJ, Fitriana F, Syahada JH, Cha SH, Chun W, Park WS, Lee SJ, Na S, Han JH, Kija N, Geodfrey S, Han ET, Todd J, Manjurano A, Han JH
Visualization: Mazigo E, Jun H, Lee WJ, Louis JM, Fitriana F, Syahada JH, Muh F, Ahmed MA, Cha SH, Chun W, Park WS, Han JH, Todd J, Manjurano A, Han JH
Writing – original draft: Mazigo E
Writing – review & editing: Han JH
Conflict of interest
The authors declare no conflict of interest related to this study.
Acknowledgments
We thank all participants, health officers, and community health workers in all places the study was conducted for their valuable contribution, cooperation, and participation during community survey and blood collection.
This work was supported by the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare (HI22C0820), “Regional Innovation Strategy (RIS)” through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (MOE) (2022RIS-005), and the National Research Foundation of Korea (NRF) funded by the Ministry of Education (RS-2023-00240627) (J-H. H.).
Supplementary Information
Supplementary material is available with this article at https://doi.org/10.3347/PHD.24077.
