In vitro immunoregulatory role of recombinant Ancylostoma ceylanicum calreticulin
Article information
Abstract
Ancylostoma ceylanicum is a zoonotic soil-derived nematode that parasitizes the intestines of humans and animals (dogs and cats), leading to malnutrition and iron-deficiency anemia. Helminth parasites secrete calreticulin (CRT), which regulates or blocks the host’s immune response. However, no data on A. ceylanicum calreticulin (Ace-CRT) are available. We investigated the biological function of recombinant Ace-CRT. (rAce-CRT) rAce-CRT showed reliable antigenicity and stimulated the proliferation of mouse splenocytes and canine peripheral blood mononuclear cells. Quantitative reverse-transcription PCR assays revealed that rAce-CRT primarily promoted the expression of T helper 2 cytokines, particularly IL-13, in canine peripheral blood lymphocytes. rAce-CRT inhibited complement-mediated sheep erythrocyte hemolysis in vitro. Our findings indicate that Ace-CRT plays an immunomodulatory role and may be a promising candidate molecule for a hookworm vaccine.
Introduction
Hookworms causes soil-transmitted nematodiasis in human and animal intestines. The buccal capsule of these blood-feeding parasites contains hook-shaped teeth for attachment to and laceration of the host’s intestinal mucosa [1], leading to malnutrition and iron-deficiency anemia. Necator americanus and Ancylostoma duodenale only infect humans, whereas A. ceylanicum and A. caninum are zoonotic that infect dogs, cats, and humans [2]. A. ceylanicum is the second most common hookworm infection in people in Southeast Asia [3], causing abdominal pain, diarrhea, malnutrition, and iron-deficiency anemia. Thus, the development of new methods to control this parasite is critical.
Calreticulin (CRT) is a multifunctional protein that primarily regulates calcium ion homeostasis, intracellular calcium concentration, and calcium storage in the endoplasmic reticulum [4]. Additionally, CRT is an intracellular chaperone for glycoproteins, engaging carbohydrate side chains via well-defined lectin domains [5], and participates in signal transduction and cell adhesion, regulating gene expression and other functions [6]. CRT protein homologs have been identified in various parasites, such as Trichinella spiralis, N. americanus, and Brugia malayi, suggesting its involvement in a variety of conserved roles [7–9]. When parasites invade the host, they secrete CRT as a defense mechanism to divert the host’s immune response (possibly generating cross-reactive antibodies to host CRT). CRT binds to host complement C1q and mannose-binding lectin [9], thereby inhibiting the classic complement pathway and activation of the lectin pathway, counteracting the host’s immune response. The binding of T. spiralis CRT to C1q was reported to inhibit C1q-involved pathogen clearance, reducing immune cell (neutrophils, eosinophils, and macrophages) recruitment to the parasite infection site and the release of reactive nitrogen intermediates and reactive oxygen intermediates [8].
The function of N. americanus CRT (Na-CRT) has been preliminarily explored [10], and our previous study reported the eukaryotic expression and immunogenicity of A. ceylanicum CRT (Ace-CRT) [11]. However, the prokaryotic expression and biological function of Ace-CRT have not been reported. Therefore, this study aimed to clone and express Ace-CRT in Escherichia coli to study its immunomodulatory role in vitro.
Materials and Methods
Ethics statement
All animal experiments were performed according to the guidelines of the Animal Welfare Law and Regulations of the Department of Health and Human Services, China and were reviewed and approved by the South China Agricultural University Animal Care and Use Committee (approval number: SYXK(Yue)2019-0136).
PCR and sequencing
The Ace-CRT gene was amplified using the specific primer pair Ace-CRT-F (5′-ACGGCCAGTGAATTCATGGCTGT-3′) and Ace-CRT-R (5′-CTATAAAAGCTTGGCG-3′) designed according to the A. ceylanicum CRT gene sequence (GenBank accession no.: EYB83200.1). Each PCR reaction (25 μl) contained 0.5 μl cDNA, 1 μl each of the forward and reverse primers, 12.5 μl 2× PrimeStar HS PCR Master Mix (TaKaRa, Dalian, China), and 10 μl ddH2O. The cycling conditions were as follows: 94°C for 5 min; 35 cycles of 94°C for 30 sec, 54.8°C for 40 sec, and 72°C for 90 sec, and 72°C for 8 min. The PCR products were visualized following 1% agarose gel electrophoresis and extracted and purified using an E.Z.N.A. Gel Extraction Kit (Omega Bio-Tek, Norcross, GA, USA). Then, the purified PCR product was ligated to a pUC-57 vector (Tsingke, Beijing, China) and transformed into E. coli DH5α competent cells (TaKaRa). Bacterial PCR was used to screen for positive clones, which were then sequenced by Sangon Biotech (Shanghai, China). The amino acid sequences of Ace-CRT were predicted using DNAMAN software 6.0. The size and position of the signal peptide in the coding sequence region of the Ace-CRT amino acid sequence were analyzed using the online software SignalP5.0 (https://services.healthtech.dtu.dk/services/SignalP-5.0/). The transmembrane domain of the Ace-CRT amino acid sequence was investigated using the online tool TMPred (https://bio.tools/TMPred). ProtScale software (https://www.expasy.org/search/protscale) was used to determine Ace-CRT hydrophobicity/hydrophilicity. The tertiary structure was predicted using the online tool SWISS-MODEL (http://swissmodel.expasy.org/).
Expression and purification of recombinant Ace-CRT (rAce-CRT)
The purified PCR products and expression vector pET28a were digested using NheI and HindIII restriction enzymes (New England Biolabs, Ipswich, UK), ligated using T4 DNA ligase, and transformed into E. coli BL21 competent cells (Sangon Biotech) for protein expression. After culture (200 rpm, 37°C) and induction for 16 h with 0.5 mM IPTG, the bacteria were collected by centrifugation and suspended in 10 ml PBS. The bacterial suspension was ultrasonicated on ice and the resulting cell lysates were centrifuged at 12,000 g for 5 min. The supernatant was transferred to a Ni-NTA affinity chromatography column (Beyotime, Shanghai, China), allowed to stand overnight at 4°C, and eluted in imidazole solution with a 0–50 mM gradient. The supernatants were precipitated. We eluted proteins after which were separately analyzed by SDS-PAGE with Coomassie brilliant blue staining (Solarbio, Beijing, China).
Western blot
Three dogs were infected by gavage with third-stage A. ceylanicum larvae. At 8 weeks after infection, blood samples (2 ml) were collected from the cephalic vein of infected dogs (positive serum) and healthy dogs (n=3, negative serum) in centrifuge tubes and refrigerated at 4°C overnight. Then, the serum was collected from each sample by centrifuging at 4,000 g for 15 min. The purified Ace-CRT protein was electrotransferred to a nitrocellulose membrane (Sangon Biotech). Western blot was performed with mouse anti-His tag antibody (1:3,000; Sangon), positive canine serum, and negative canine serum as primary antibodies. Horseradish peroxidase-conjugated goat anti-mouse and rabbit anti-dog antibodies (1:3,000; Sangon) were used as secondary antibodies. The specificity and antigenicity of the recombinant protein were tested using a DAB western blot detection kit (Solarbio).
MTT assay
We sacrificed 10 healthy 6-week-old BALB/c mice and soaked them in alcohol for disinfection before removing the spleen from the abdominal cavity, crushing it into pieces, and filtering it through a 200-mesh sieve to collect the primary splenocytes. Then, RPMI-1640 medium supplemented with 10% fetal bovine serum was added to the isolated splenocytes and centrifuged at 2,400 g for 5 min. After decanting the supernatant, splenocyte washing and centrifugation were repeated. Then, the cell pellets were harvested and resuspended in RPMI-1640 medium, and the number of viable cells was counted following trypan blue staining (Sangon Biotech). Samples with >95% viability were selected for subsequent analysis.
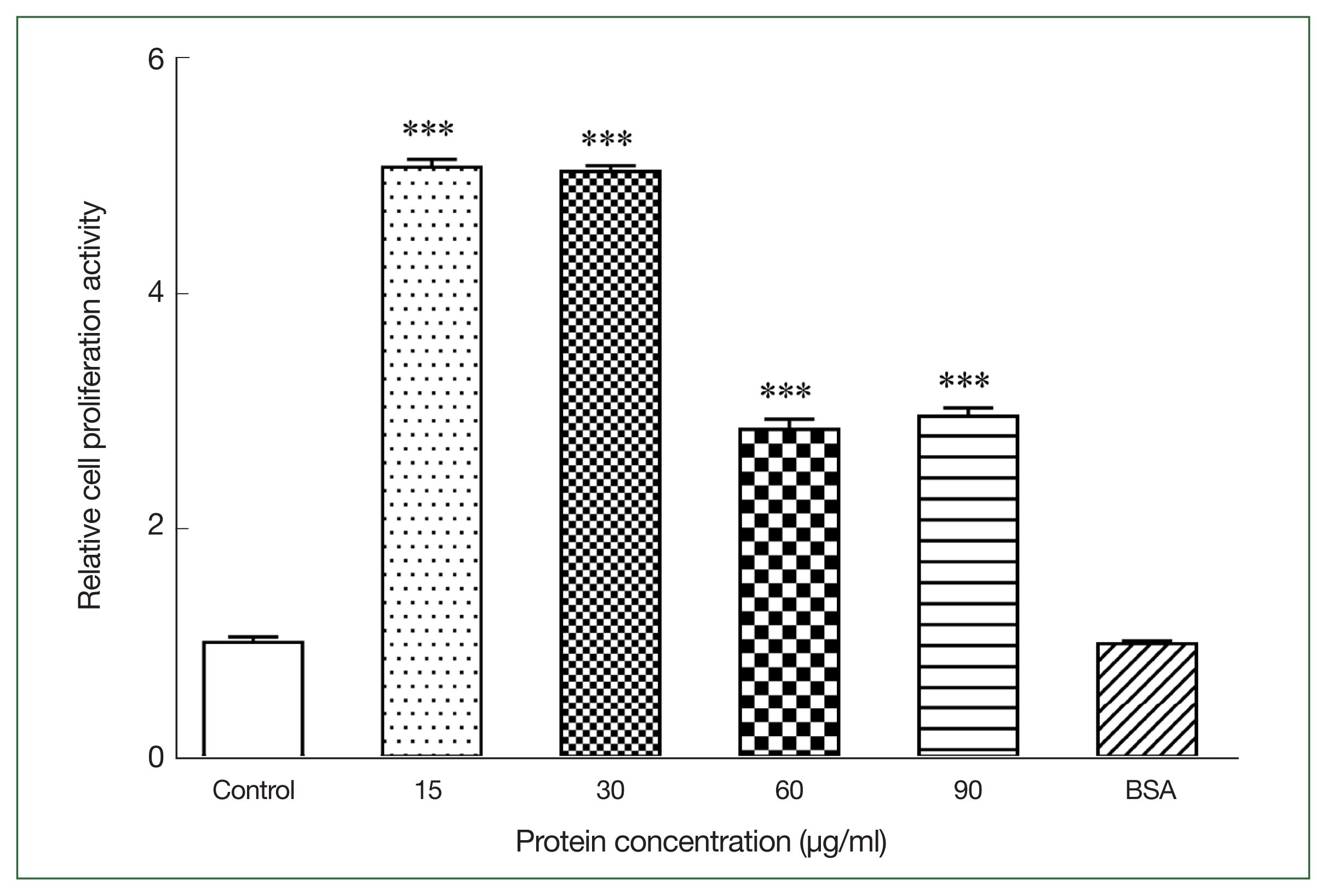
The mouse splenocytes (2×103) were added to each well of a 96-well plate and cultured for 24 h in a 5% CO2 cell culture incubator at 37°C. After stabilization, 100 μl of rAce-CRT at concentrations of 15, 30, 60, and 90 μg/ml and BSA (negative control) were added to each well and placed in a 5% CO2 cell culture incubator (37°C, 24 h). Finally, the absorbance values at 570 nm were measured in each group following the addition of MTT.
Cytokine expression in peripheral blood mononuclear cells (PBMCs)
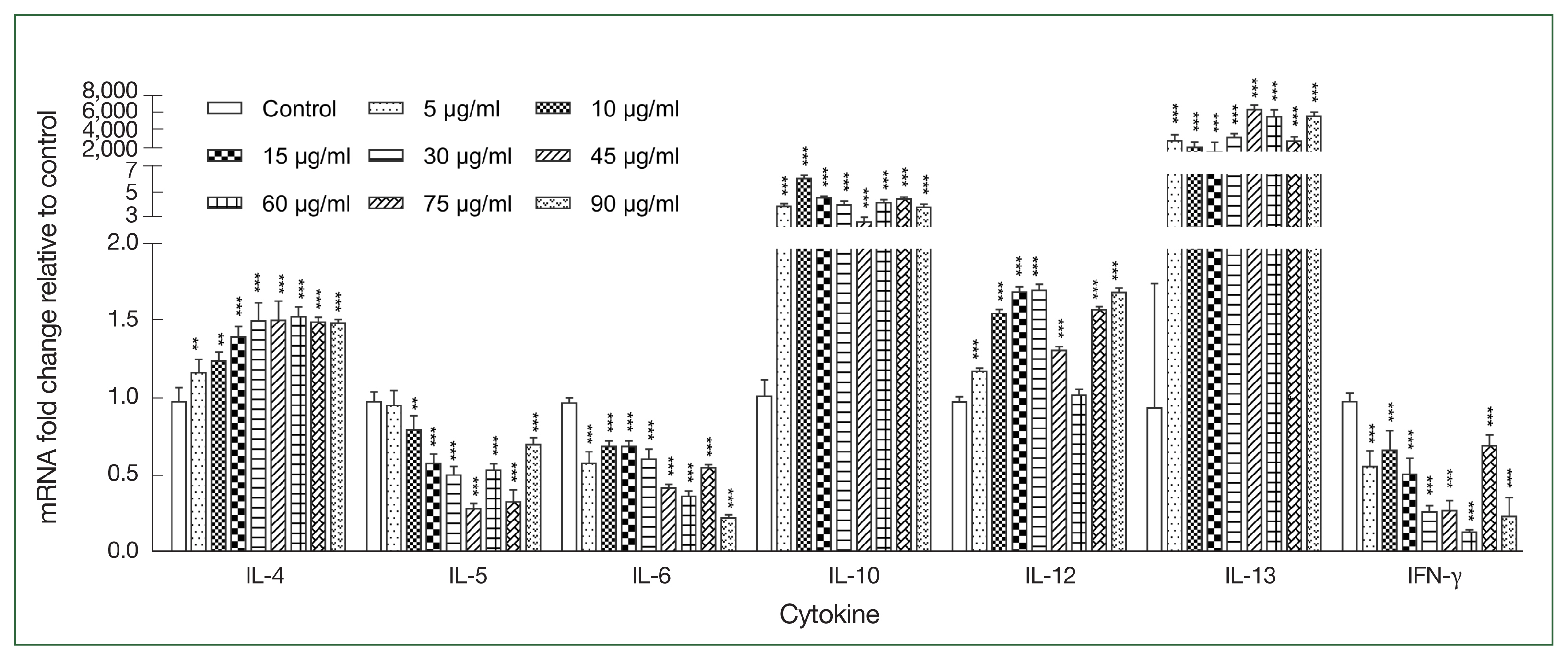
We used a Lymphocyte Isolation Kit (TBD, Tianjin, China) to isolate canine PBMCs from a 2-ml sample of anticoagulated blood collected from a healthy dog, as described previously [12]. After lymphocyte growth was stabilized, the experimental groups (with purified protein), blank control, and negative control (BSA) were prepared. Eight working concentrations of purified protein (5, 10, 15, 30, 45, 60, 75, and 90 μg/ml) were used in the experimental groups, with 3 repetitions per group. The prepared plate was incubated in a 5% CO2 cell culture incubator (37°C, 24 h). Then, the cell suspension was centrifuged, and the precipitates were collected for extraction of PBMC total RNA using an E.Z.N.A. Total RNA Kit (Omega Bio-Tek) according to the manufacturer’s instructions. A microplate reader was used to assess the RNA quality (optical density [OD]260 nm/OD280 nm≈2.0), and agarose gel electrophoresis was performed to determine RNA integrity. The recovered PBMC total RNA was used to synthesize first-strand cDNA by reverse transcription using HiScript III RT SuperMix for qPCR (+gDNA wiper) (Vazyme, Nanjing, China) according to the manufacturer’s protocol. Fluorescence quantitative reverse-transcription PCR was performed using a LightCycler 96 Instrument (Roche, Basel, Switzerland) to detect cytokine expression in PBMCs stimulated with different concentrations of Ace-CRT. Supplementary Table S1 shows the sequences and lengths of the real-time PCR primers used to target canine 18S rRNA and the genes for cytokines IL-4, IL-5, IL-6, IL-10, IL-12, IL-13, IL-23, and IFN-γ. Each reaction (10 μl) comprised 5 μl ChamQ Universal SYBR qPCR Master Mix (Vazyme), 0.4 μl of each forward and reverse primer, 1 μl cDNA, and 3.2 μl ddH2O. The qPCR reaction conditions were 50 cycles of 95°C for 1 min, 95°C for 10 sec, and 60°C for 30 sec. 18S rRNA was used as the internal reference gene, and each sample was assayed in triplicate. The relative cytokine expression levels were calculated using the 2-ΔΔCT method [21].
Hemolysis assay
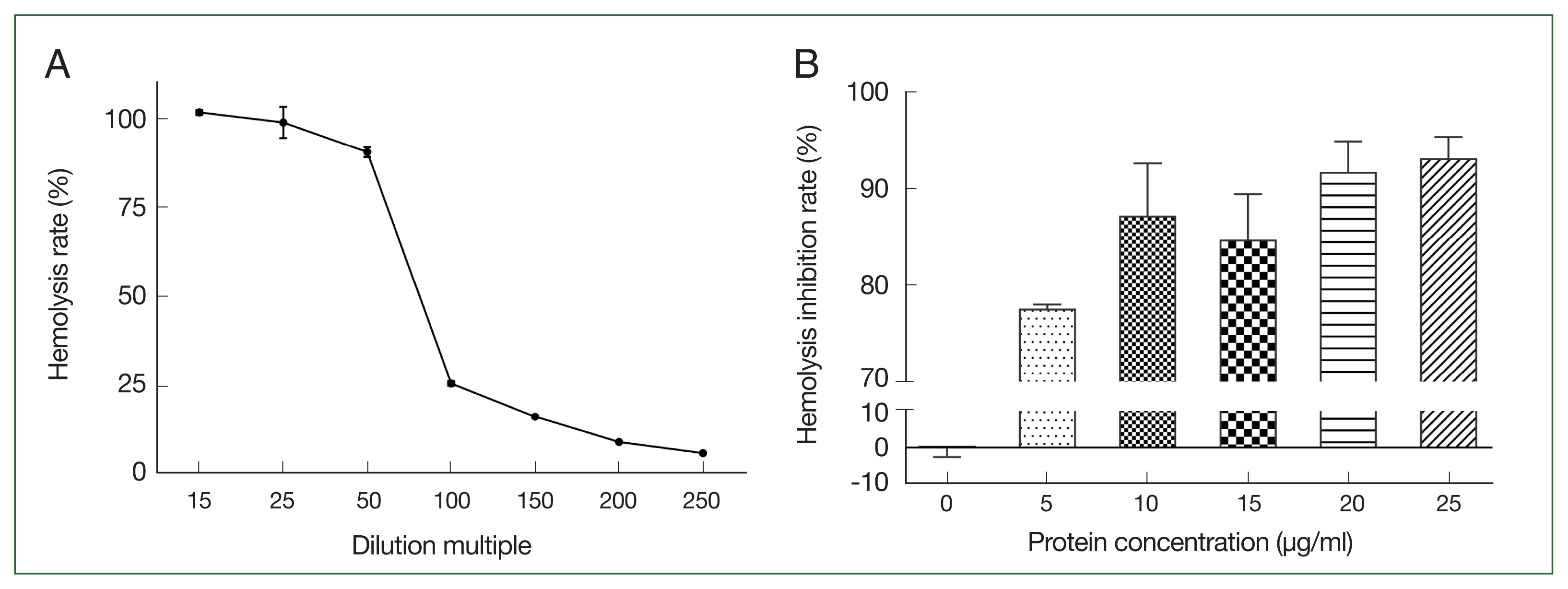
We performed a hemolysis assay with a slight modification of the previous method [22]. Briefly, whole blood from a healthy dog was collected into a clean centrifuge tube and left to stand for 1 h at room temperature, followed by 2 h at 4°C. Then, the sample was centrifuged at 6,000 g for 10 min at 4°C to collect the serum. Next, the serum was diluted to 1:2, 1:5, 1:10, 1:20, 1:40, 1:80, and 1:160 with barbitone buffer solution (BBS-GM) to determine the optimal serum dilution. Each serum dilution was mixed with sensitized sheep erythrocytes and BBS-GM (200 μl each) for the hemolysis test, with 3 replicates per group. A mixture of sheep erythrocytes (2%, 100 μl) and ultrapure water (500 μl) was considered the complete hemolysis group, and its absorbance was used as the standard to calculate the hemolysis rate. Next, the optimal dilution of canine serum (200 μl) was mixed with 200 μl of rAce-CRT at different concentrations (5, 10, 15, and 25 μg/ml). The serum/rAce-CRT suspensions were then gently mixed with 200 μl of sensitized sheep erythrocytes, incubated at 37°C for 30 min in a water bath, and centrifuged at 2,400 g for 10 min. The supernatant (150 μl) was collected from each group to measure the absorbance at 405 nm (A1). BBS-GM, rAce-CRT, and sensitized sheep red blood cells were used as the control group to measure absorbance at 405 nm (A2). BBS-GM, canine serum, and sensitized sheep erythrocytes (200 μl each) were used as the complement group for measuring the absorbance at 405 nm (A0). The hemolysis inhibition rate was calculated as follows: (1− [A1−A2]/A0)×100 and expressed as a percentage.
Statistical analysis
Data were expressed as the mean±SD. All results were analyzed using SPSS 22.0 (SPSS, Chicago, IL, USA) and GraphPad Prism 6.2 (GraphPad, San Diego, CA, USA). A P-value <0.05 was considered statistically significant.
Results
Recombinant Ace-CRT
Using A. ceylanicum cDNA as the template, PCR targeting Ace-CRT yielded a PCR product of approximately 1,200 bp (Supplementary Fig. S1A). Following cloning, PCR of the bacterial suspension resulted in amplified bands corresponding to the expected fragment size (Supplementary Fig. S1B). After double-enzyme digestion of the recombinant plasmid pET28a-Ace-CRT, a fragment of approximately 1,200 bp (Supplementary Fig. S1C) was obtained, consistent with the expected target size.
Recombinant protein analysis by western blot
We used SDS-PAGE to analyze the bacterial culture (before and after expression induction), after which purified protein. The protein of interest was predominantly expressed in the supernatant, and optimal purification was achieved using 50 mm imidazole. The size of the recombinant protein was approximately 50 kDa (Supplementary Fig. S2), consistent with the expected size of the target protein. Western blot analysis revealed that the recombinant protein was recognized by anti-His tag antibodies, showing a specific band at 50 kDa (Supplementary Fig. S3A). The recombinant protein was also recognized by positive canine serum, showing a distinct band at 50 kDa. No reaction bands were observed with negative canine serum (Supplementary Fig. S3B), indicating reliable antigenicity of the recombinant protein.
Proliferation of mouse spleen cells by rAce-CRT
We measured the OD570 nm of mouse primary splenocytes after coculturing with different rAce-CRT concentrations to assess the effect of rAce-CRT on splenocyte proliferation. rAce-CRT significantly stimulated mouse primary splenocyte proliferation (P<0.05) (Fig. 1). The rate of splenocyte proliferation following coculture with 15, 30, 60, and 90 μg/ml rAce-CRT was significantly increased (P<0.05) and was highest in the coculture with 15 and 30 μg/mL rAce-CRT (Fig. 1). These findings suggest that Ace-CRT promotes immune cell proliferation and plays an immunomodulatory role.
Cytokine expression in infected dogs
After stimulating canine PBMCs with different concentrations of rAce-CRT, the expression levels of IL-4, IL-10, IL-12, and IL-13 were increasecl compared with the expression levels in the control group (P<0.05), but the difference between the rAce-CRT-stimulated group and the control group was only significant for IL-13 (P<0.05). In contrast, the expression levels of IL-5, IL-6, and IFN-γ decreased in the rAce-CRT-stimulated group compared with the expression levels in the control group (Fig. 2).
Hemolytic assay using A. ceylanicum-positive canine serum
Increasing the canine serum dilution ratio resulted in a gradual reduction in the rate of hemolysis of sensitized sheep erythrocytes. The hemolysis rate was 90.7% when the serum dilution ratio was 1:50 (Fig. 3A). Compared with the control group, the different concentrations of rAce-CRT inhibited hemolysis of sensitized sheep erythrocytes. The hemolysis inhibition rate increased with an increasing protein concentration (Fig. 3B).
Discussion
Benzimidazoles are currently the main treatment option for deworming and controlling hookworm disease [23]. However, this benzimidazoles measure cannot prevent hookworm reinfection, and the parasite is prone to drug resistance [24]. There is an urgent need to identify a candidate vaccine molecule for more effective prevention and control measures. CRT is a multifunctional protein with anti-inflammatory, hemostatic, and immune potential. Pritchard et al. [10] reported that N. americanus-CRT (Na-CRT) directly induced human basophils and mast cells to produce type II cytokines, causing allergic reactions. When purified recombinant protein was combined with human complement protein C1q, the hemolytic function of C1q was significantly inhibited [25], indicating that Na-CRT plays an immunomodulatory role. Additionally, upon N. americanus larval challenge, mice vaccinated with Na-CRT showed a 43–49% reduction rate in worms recovered from the lungs compared with the control group, indicating the vaccination potential of Na-CRT [26].
The length of the cDNA encoding Ace-CRT was 1,224 bp, and the recombinant protein size was approximately 50 kDa. Sequencing results revealed that the Ace-CRT gene was 1,224 bp in length (GenBank accession no.: OM280324), encoding an open reading frame of 407 amino acids. Analysis of the amino acid sequence of Ace-CRT revealed that the average hydrophilicity coefficient of the protein was <0, indicating that Ace-CRT was hydrophilic. Furthermore, Ace-CRT was determined to have a transmembrane domain, and the first 20 amino acids of the protein represented a signal peptide sequence. The quality assessment value of the model of the protein’s 3D structure was 0.66, indicating good modeling quality. SDS-PAGE analysis of the pellet and supernatant showed the expression of large quantities of the recombinant protein in the supernatant, which was very convenient for subsequent purification and consistent with the finding that the protein was hydrophilic. The recombinant protein bound to His tag antibodies and was recognized by positive canine serum, indicating good antigenicity. Coincubation of rAce-CRT with different concentrations of mouse primary splenocytes revealed that rAce-CRT strongly stimulated lymphocyte proliferation. This finding suggested that Ace-CRT promoted immune cell proliferation.
Cytokines are polypeptide molecules synthesized and secreted by immune cells that regulate cell differentiation and immune function [27]. The expression levels of cytokines IL-4, IL-10, IL-12, and especially IL-13 were significantly increased, whereas the expression levels of IL-5, IL-6, and IFN-γ were decreased in canine PBMCs stimulated by Ace-CRT recombinant protein. These results were consistent with cytokine expression pattern in mice immunized with the eukaryotic plasmid [11]. IL-4 is a product of T cells that induces T helper cells (Th; naive CD4+ T cells) to differentiate into the Th2 subset of effector cells. This subset is characterized by the stereotypic production of a suite of cytokines, including IL-4, IL-5, IL-10, and IL-13, by committed Th2 cells [28]. IL-4 plays a crucial role in primary and secondary parasitic infections, contributing to host resistance against parasite invasion [29]. IL-13 is a typical Th2-type cytokine with an anti-inflammatory effect [30] that inhibits macrophage activation and stimulates B cell proliferation. IL-12 is secreted by antigen-presenting cells and promotes the differentiation of Th0 to Th1 cells, which primarily resist intracellular pathogen infection but cannot effectively counteract hookworms. However, IL-5 stimulates eosinophil maturation and induces Th2 inflammatory responses [31]. IL-6 primarily functions as an enhancer of T cell proliferation [29], and IFN-γ antagonizes IL-4, activates macrophages, and inhibits Th2 cell proliferation. Therefore, we concluded that CRT of A. ceylanicum might be a main contributor to the dominance of the Th2 immune response in the host to resist parasite infection, similar to the conclusion of research on Taenia solium CRT [32].
C1q is the recognition protein of the classical complement pathway and the main connecting protein between innate immunity driven by the classical complement pathway and acquired immunity mediated by IgG or IgM [7]. In this study, we used the complement system of canine serum and measured the complement activity to detect the reaction between rAce-CRT and complement. The results showed that rAce-CRT inhibited the hemolysis of sensitized sheep erythrocytes. Similarly, Na-CRT was reported to bind to human C1q and inhibit C1q-mediated hemolysis [25], and Haemonchus contortus CRT was found to bind to C1q and inhibit C1q-mediated erythrocyte cleavage [33]. These observations were conducive to host survival, consistent with the results of this study.
In summary, this is the first report of the cloning of cDNA encoding Ace-CRT. A recombinant plasmid was constructed, and the prokaryotic expression, purification, and function of the recombinant proteins were explored. rAce-CRT showed good antigenicity and stimulated the proliferation of mouse spleen cells and canine PBMCs. Ace-CRT stimulated the secretion of Th2 cytokines and inhibited complement-mediated hemolysis. These findings lay the foundation for screening candidate molecules for a vaccine against A. ceylanicum.
Supplementary Information
Supplementary Fig. S1 Amplification of Ace-CRT gene, bacterial fluid PCR, and recombinant plasmid enzyme-digestion. (A) Ace-CRT gene. M, DL2000; lane 1, Full-length gene fragment; lane 2, Gene fragment except signal peptide. (B) bacterial fluid PCR. M, DL2000; lane 1, Monoclonal colonies with full-length gene; lane 2, Monoclonal colonies with gene fragment except signal peptide. (C) Enzyme-digested recombinant plasmid. M, DL5000; lane 1, Enzyme-digested product of pET28a-Ace-CRT recombinant plasmid.
Supplementary Fig. S2. SDS-PAGE analysis of purified Ace-CRT recombinant protein. M, Protein molecular weight marker; lane 1, Uninduced recombinant bacteria; lane 2, Precipitation of induced recombinant bacteria; lane 3, Supernatant of induced recombinant bacteria; lane 4, 0 mM imidazole eluate; lane 5, 2 mM imidazole eluate; lane 6, 10 mM imidazole eluent; lane 7, 50 mM imidazole eluent.
Supplementary Fig. S3. Western blot analysis of Ace-CRT recombinant protein. (A) Identification of recombinant protein . M, Protein molecular weight marker; lane 1, rAce-CRT reacted with anti-His tag antibody. (B) Antigenic specificity of recombinant protein. M, Protein molecular weight marker; lane 1, The rAce-CRT reacted with positive serum of infected dogs; lane 2, rAce-CRT reacted with negative serum of healthy dogs; lane 3, Negative control.
phd-23108-Supplementary-Figs.docxPrimers used for the quantitative real-time PCR
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (Grant No. 31672541) and the Science and Technology Planning Project of Guangdong Province, China (Grant No. 2014A020214005).
Notes
Data curation: Zhuang T
Investigation: Zhuang T
Methodology: Zhuang T, Abuzeid AMI, Chen X, Zhu S
Project administration: Li G
Supervision: Li G
Writing – original draft: Zhuang T, Abuzeid AMI, Chen X, Zhu S
Writing – review & editing: Abuzeid AMI, Li G
The authors declare that they have no conflicts of interest.