The genera
Opisthorchis Blanchard, 1895;
Clonorchis (Cobbold, 1875) Looss, 1907;
Metorchis (Cobbold, 1860) Looss, 1899; and
Amphimerus Barker, 1911 encompass 4 significant groups of small liver flukes that infect humans globally [
1,
2]. Among these,
Opisthorchis is of particular public health concern, as 2 of its species,
Opisthorchis viverrini (Poirier, 1886) Stiles and Hassall, 1896 and
Opisthorchis felineus (Rivolta, 1884) Blanchard, 1895, are human pathogens associated with severe diseases, including cholangiocarcinoma, in the Greater Mekong Subregion (
Supplementary Fig. 1S) and Russia-Eastern Europe, respectively [
1,
2]. In
O. viverrini, high-endemic areas are documented in Thailand and Laos, while low to medium-endemic regions are reported in Vietnam, Cambodia, Myanmar, and Malaysia [
1,
2]. Remarkably, Thailand alone experiences an estimated 27,500 annual deaths from liver cancer attributable to opisthorchiasis, prompting nationwide control initiatives [
3].
The genus
Opisthorchis encompasses at least 54 nominal species with reported hosts spanning fish, reptiles, birds, and mammals [
4–
6]. Within this diversity, approximately 30 species have been described from birds and 11 from mammals, although taxonomic revisions have resulted in synonymizations and transfers to other genera [
4–
7]. In the Greater Mekong Subregion, endemic for the zoonotic species
O. viverrini, the presence of other
Opisthorchis species has been documented [
5,
8,
9]. Specifically, in Vietnam,
Opisthorchis cheelis Lal, 1939,
Opisthorchis longissimus (Linstow, 1883),
Opisthorchis parageminus Oshmarin, 1970, and
Opisthorchis sp. BD2013 Dao et al. 2017 have been reported from wild birds and ducks [
8,
9]. In Vientiane, Laos, metacercariae of
Opisthorchis lobatus (Bilqees et al. 2003) Scholz, 2008, originally described as
Neometorchis lobatum from ducks in Pakistan, were identified in snakehead fish, with adult flukes subsequently obtained from experimental hamster infections [
10].
Genetic analyses conducted in Thailand have revealed that
O. viverrini distributed throughout the Greater Mekong Subregion represents a species complex (
O. viverrini sensu lato) [
11]. These studies identified 2 distinct populations of putative
O. viverrini, 1 predominantly found in humans (human-specific population) and the other primarily in cats (cat-specific population) [
12,
13]. The cat-specific population exhibited 99.5%–100% sequence identity with flukes transmitted by snakehead fish in Khon Kaen, Thailand, based on the mitochondrial cytochrome
c oxidase subunit I (
cox1) and NADH dehydrogenase subunit I (
nd1) sequences [
12,
13]. Furthermore, phylogenetic analyses placed this clade alongside
O. lobatus reported from Vientiane, Laos, as depicted in
Fig. 1 of Agustina et al. [
13].
In Myanmar, human infections with
O. viverrini were initially identified through molecular analysis of fecal eggs in the Bago Region (north of Yangon), Mon State (east of Yangon), and the Yangon Region [
14]. Subsequent studies in the Yangon Region by our group reported a low-grade
O. viverrini endemicity, with a fecal egg-positive rate of 0.7% (14/2,057 individuals) and successful recovery of an adult
O. viverrini fluke from an egg-positive volunteer following praziquantel treatment and purging [
15]. Metacercariae found in cyprinid fish (
Puntius brevis) from the Bago Region and adult flukes obtained from experimental hamsters were identified as
O. viverrini based on morphological and molecular data, including internal transcribed spacer 2 and
cox1 sequences [
16]. Furthermore, metacercariae morphologically identified as
O. viverrini were detected in cyprinid fish
Cyclocheilichthys repasson from Tachileik, located in the Mekong Region of Myanmar (along the eastern border with Laos and Thailand) [
17]. However, reports on other
Opisthorchis species in avian or mammalian hosts within Myanmar remained absent. We recently identified a liver fluke species infecting snakehead fish in the Yangon Region. Adult flukes recovered from an experimentally infected hamster exhibited morphological similarity to
O. viverrini but also displayed remarkable differences. In this study, we provisionally designated our specimens as an
O. viverrini-like liver fluke and detailed their morphological characteristics.
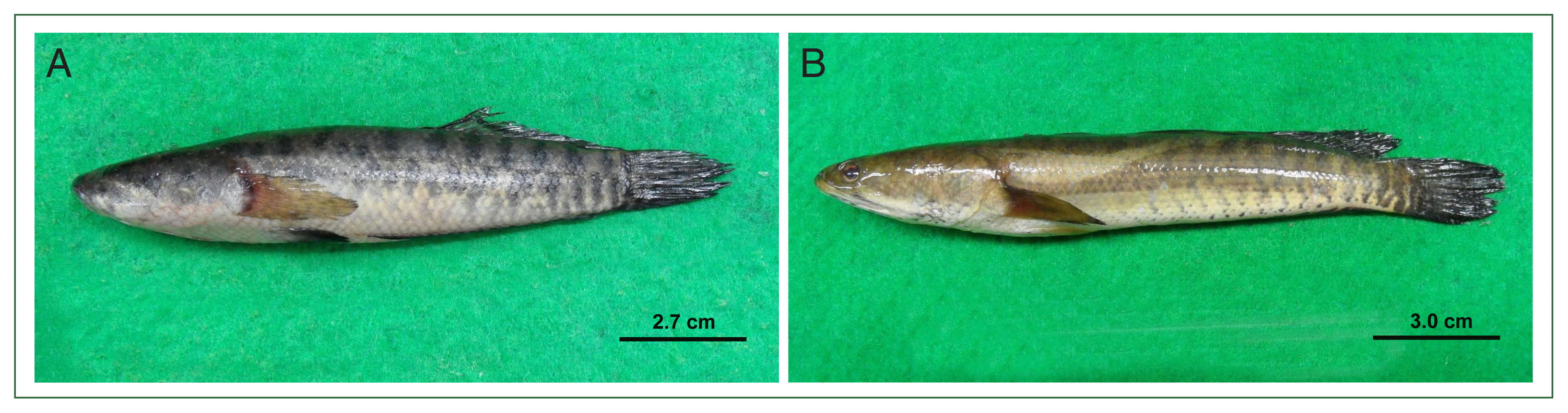
Metacercariae (160 in total number) were isolated from 2 snakehead fish species
Channa lucius (
n=5; 100% prevalence, 122 metacercariae) and
Channa striata (
n=29; 3.4% prevalence, 38 metacercariae) (
Fig. 1A, B), purchased from fish markets in North Dagon, Yangon between 2015 and 2016. The metacercariae were isolated using the muscle compression and digestion techniques. One hundred metacercariae were orally administered to a hamster via a gavage needle. On day 50 post-infection, the hamster was euthanized, and its entire liver was excised. The liver tissue was dissected into small pieces and gently compressed to extract adult flukes (
n=20) from the bile ducts, which were then collected in 0.85% saline. The animal experiment was approved by the Institutional Animal Care and Use Committee of Gyeongsang National University College of Medicine, Jinju, Korea (No. 2016-18). Adult flukes were fixed in 10% neutral formalin and stained with Semichon’s acetocarmine. Briefly, formalin-fixed specimens were washed in water, stained overnight with acetocarmine, and destained with 1% acid alcohol. Specimens were subsequently dehydrated through a graded ethanol series (70%, 80%, 90%, 95%, and 100%), cleared in xylene, and mounted in Permount. Measurements were performed on 10 well-preserved and stained specimens and are expressed in millimeters (mm) unless otherwise specified.
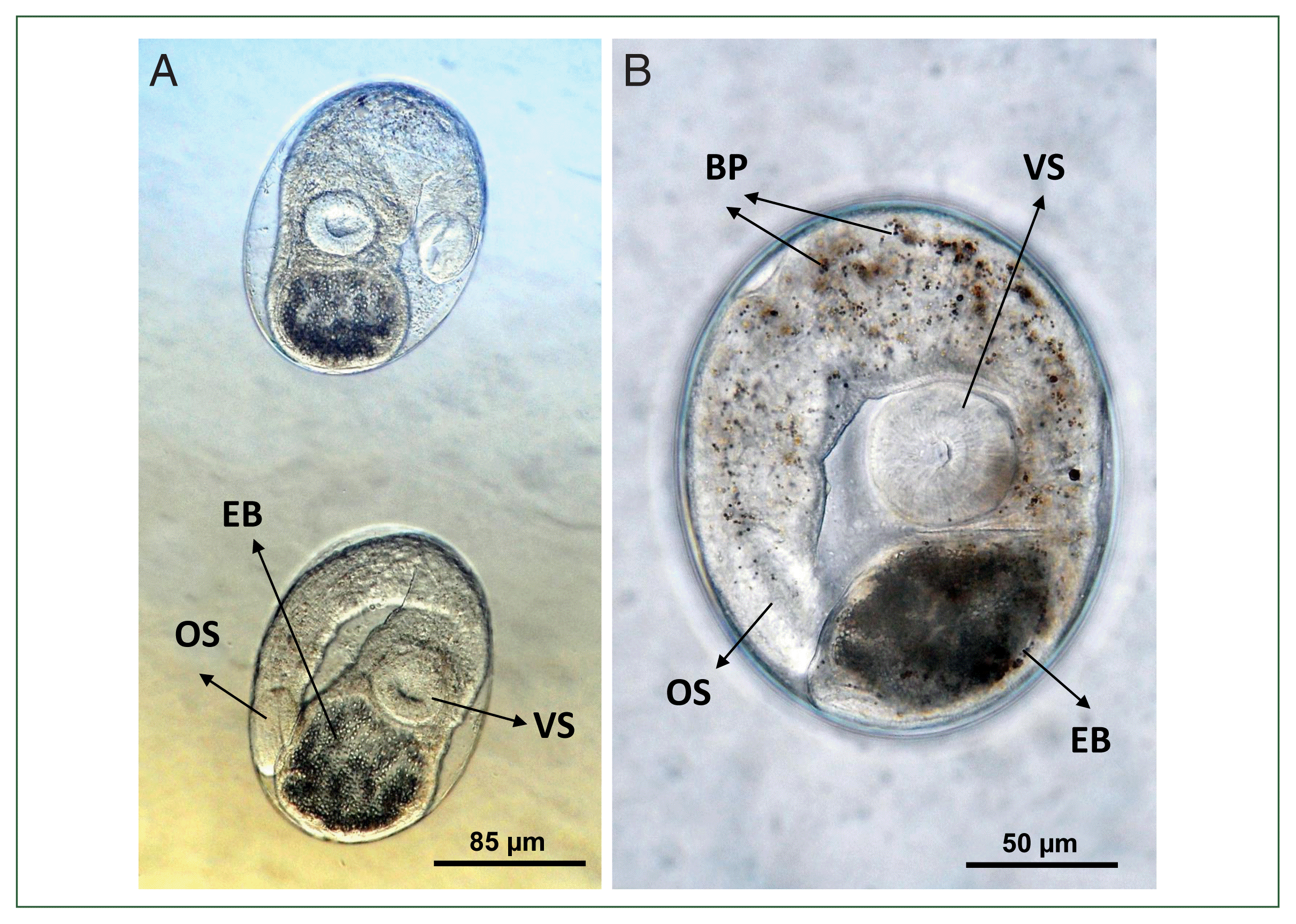
Metacercariae were elliptical, measuring 150–188×98–140 μm (average 165×122 μm,
n=10) (
Fig. 2A, B), which are smaller than the reported measurements for
O. viverrini (200×170 μm [
18]). They exhibited a double-layered cyst wall, consisting of a thin inner layer and a thick outer layer, and were equipped with an oral sucker, a ventral sucker, and a large excretory bladder. The oral sucker was nearly rounded and subterminal; the prepharynx was rudimentary or absent; the pharynx was round or oval; and the esophagus was relatively elongated. The ventral sucker was slightly larger than the oral sucker. The excretory bladder was round to elliptical, containing numerous small excretory granules. Brownish pigment granules were observed scattered throughout the body. The metacercariae were located within the musculature of the fish.
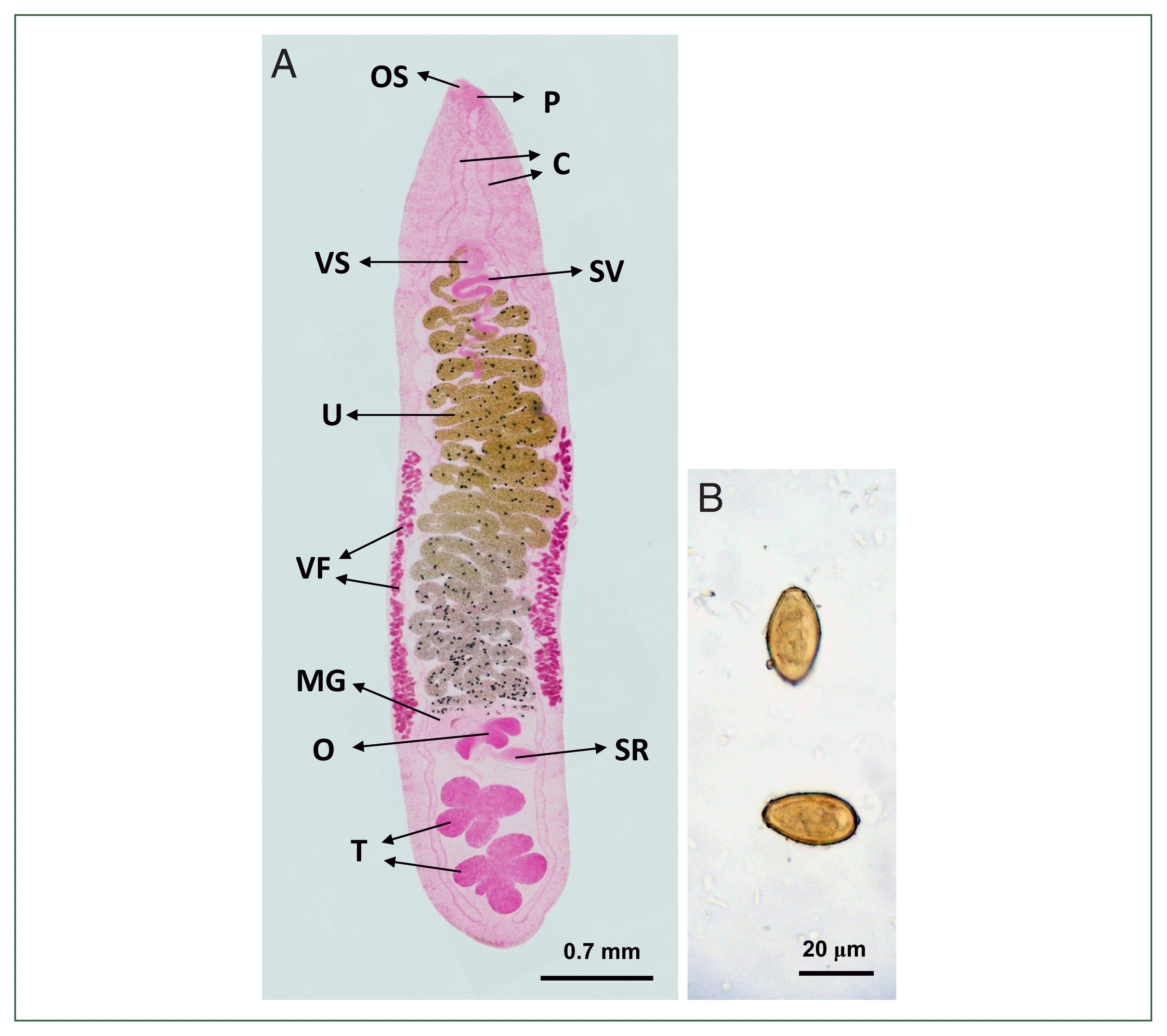
Adult flukes (
n=10) were lanceolate or elongated leaf-like, measuring 3.8–6.0 (average 5.1) mm long and 1.1–1.2 (1.2) mm wide (
Fig. 3A). The body tegument was smooth, lacking spines. The oral sucker was oval and subterminally located on the anteroventral side, measuring 0.08–0.11 (0.10) mm by 0.12–0.15 (0.13) mm. The prepharynx was absent, and the pharynx measured 0.10–0.11 (0.10) mm by 0.10–0.13 (0.11) mm. The esophagus was longer than the pharynx, with the length ranging from 0.13–0.29 (0.20) mm, and connected to 2 intestinal ceca. Two ceca were long, occasionally inflated, and extended laterally between the uterus and vitelline follicles to the posterior extremity. The ventral sucker, located along the median line of the body, was globular and measured 0.12–0.15 (0.13) mm in diameter, being slightly larger than the oral sucker. The ovary was 3-lobed, 0.29–0.39 (0.36) mm by 0.38–0.54 (0.48) mm, and connected to a large sac-like seminal receptacle of 0.42–0.55 (0.48) mm by 0.20–0.34 (0.28) mm in size. The Mehlis’ gland was transversely elongated between the ovary and the uterine tubule. The uterine tubule was well-developed with more than 20 windings, occupied 50%–60% of the body length from the Mehlis’ gland to the genital opening near the ventral sucker, but did not extend anterior to it. The 2 testes were positioned slightly diagonal rather than tandem near the posterior end of the body between the 2 ceca and were asymmetrical and deeply lobed. The anterior testis, measuring 0.28–0.49 (0.39)×0.54–0.67 (0.59) mm, exhibited 4 lobes. The posterior testis, measuring 0.33–0.49 (0.42)×0.54–0.57 (0.65) mm, exhibited 5 lobes. The seminal vesicle was elongated in a spiral form with >5 windings and located posteromedian to the ventral sucker. The genital opening, situated just anterior to the ventral sucker, contained separate male and female openings. Vitelline glands consisted of numerous follicles, not forming distinct groups, and extended from the level of the ventral sucker to the Mehlis’ gland or the anterior margin of the ovary. The excretory bladder was S-shaped and located between the ovary and the 2 testes. The eggs were ovoid to elliptical, measuring 26.3–27.5 (26.9) μm in length and 13.8–15.0 (14.6) μm in width (
n=10) (
Fig. 3B).
Our specimens were characterized by the following morphological features: an oral sucker slightly smaller than the ventral sucker, a 3-lobed ovary connected to a unipartite sac-like seminal receptacle, a long, winding uterus not extending anterior to the ventral sucker, vitelline follicles extending from the ventral sucker to the anterior ovary level, and 2 diagonally positioned testes, deeply lobed with 4-lobes (anterior) and 5-lobes (posterior). Among the 41 avian and mammalian
Opisthorchis species, our specimens greatly resembled
O. viverrini. However, notable differences were observed; a more spiraled seminal vesicle with more than 5 windings (less spiraled in
O. viverrini), a longer uterus occupying 50%–60% of the body length (compared to less than 49% in
O. viverrini), less distinct grouping of vitelline follicles (7–8 apparent groups per side in
O. viverrini), vitelline follicles not extending beyond the anterior ovary level (reaching the posterior ovary or anterior testis in
O. viverrini), and smaller metacercariae (
Table 1) [
10,
18,
19]. Furthermore, our specimens differed from
O. lobatus [
10], which also uses snakehead fish as an intermediate host in Laos, by possessing a spiraled seminal vesicle (irregularly coiled in
O. lobatus), less grouped vitellaria (forming 7–8 apparent groups per side in
O. lobatus), a longer uterus, and diagonally positioned, 4-lobed (anterior) or 5-lobed (posterior) testes (tandem, irregularly lobed testes in
O. lobatus) (
Table 1) [
10,
18,
19]. Therefore, we classified our specimens as an
O. viverrini-like liver fluke from Myanmar.
Regarding fish intermediate hosts of
O. viverrini, approximately 50 cyprinid species from 28 genera and 3 non-cyprinid species from 3 genera have been documented [
11]. Among the non-cyprinid hosts, snakehead fish (
Channa spp.), including
C. striata, have been reported as hosts for
O. viverrini or
O. viverrini-like liver fluke in Vietnam, Laos, Thailand, Myanmar, Cambodia, China, Indonesia, Malaysia, and the Philippines [
11]. However, the precise identification of
Opisthorchis spp. metacercariae in snakehead fish has presented significant challenges [
9,
20]. For example, in southern Vietnam, opisthorchiid metacercariae were detected in snakehead fish from An Giang Province, but species identification was not achieved due to the lack of successful adult fluke recovery via animal experimental infection [
20]. In Laos,
O. lobatus metacercariae were identified in snakehead fish from Vientiane, and experimental hamster infections yielded adult flukes morphologically assigned to
O. lobatus [
10]. In Thailand,
O. viverrini-like metacercariae from snakehead fish in Khon Kaen were confirmed as the infective stages of the cat-specific
O. viverrini population [
13]. In Myanmar,
O. viverrini-like metacercariae from snakehead fish in Yangon City could not be specifically identified [
21]. These findings collectively suggested that snakehead fish in the Greater Mekong Subregion are infected with a variety of opisthorchiid fluke metacercariae, including cat-specific
O. viverrini,
O. lobatus, and other
Opisthorchis spp., necessitating further investigation.
While hamsters proved susceptible to infection with
Opisthorchis flukes described herein, the definitive host of these flukes remains uncertain. Specifically, the possibility that these flukes represent an avian liver fluke cannot be completely excluded. For instance,
O. lobatus, initially identified as a parasite of poultry (
Anas sp.) in Pakistan, was subsequently shown to infect experimental hamsters in Laos [
10]. However, recent molecular investigations suggest that
O. lobatus may be a member of the
O. viverrini species complex, potentially corresponding to the cat-specific population of
O. viverrini [
13]. This hypothesis requires further empirical validation.
Molecular analyses have proven invaluable in elucidating the phylogenetic relationships among various genetic clades of opisthorchiid liver flukes within the Greater Mekong Subregion [
12,
13]. Regrettably, in the present study, molecular analyses could not be conducted due to an insufficient number of adult specimens recovered from the experimental hamster. We intend to address this limitation by recovering an adequate number of adult flukes through further animal experimental studies using metacercariae from snakehead fish in Myanmar, thereby enabling subsequent molecular analyses.
In conclusion, the opisthorchiid metacercariae identified in snakehead fish from the Yangon Region, Myanmar were provisionally designated as an “O. viverrini-like liver fluke” based on the distinct morphology of adult flukes recovered from an experimental hamster. However, further investigations of natural definitive hosts and comprehensive molecular analyses are needed to determine the precise taxonomic status of this form. Specifically, it is crucial to ascertain whether these specimens represent intraspecific variation within O. viverrini or a distinct Opisthorchis species.