In recent years, pet ownership in Korea has increased, with more households owning cats and a significant rise in stray cat adoptions [1]. Stray cats often live in unsanitary environments, raising concerns about their exposure to infectious agents and the potential transmission of diseases to humans [2]. As human interactions with stray cats become more frequent, understanding their infectious status as potential carriers of zoonotic pathogens is crucial.
Feline parasitic infections are primarily caused by helminths such as Toxocara cati and Ancylostoma sp., as well as protozoan parasites like Toxoplasma gondii and Giardia sp. [2]. Additionally, various zoonotic intestinal trematodes have been identified in stray cats living in riverside areas of Korea [3]. However, direct transmission of these zoonotic pathogens from cats to humans has not been clearly established. Research on zoonotic parasite infections in stray cats is essential for understanding transmission dynamics and their significance from a One Health perspective. This study aimed to assess the prevalence of intestinal parasitic infections, with a particular focus on zoonotic pathogens, among stray cats in Gimpo-si (city), Gyeonggi-do (province), Korea.
All animal experiments were approved by the Institutional Animal Care and Use Committee of the Korea Association of Health Promotion under approval number KAHP-IACUC-2021001. A total of 237 stray cats from Gimpo-si, Gyeonggi-do, Korea, were captured between August and November 2021 as part of the government-operated trap-neuter-return program. The cats were transported to Sion Animal Hospital for neutering surgery. Blood samples were collected from all 237 cats during surgery, while 101 fecal samples were obtained from the cages of recovering cats. After the procedures, the cats were released back to their original locations. Of the 237 cats, 131 (55.3%) were female, and 106 (44.7%) were male. The collected fecal and blood samples were analyzed using stool examination, molecular techniques, and serological methods to determine the prevalence of parasitic infections in this study.
A total of 101 fecal samples were processed using the formalin-ether sedimentation method, with slight modifications based on the World Health Organization standard guidelines. The samples were examined under a microscope for helminth eggs. First, 1 g of fecal sample was mixed with 10–15 ml of water to create a suspension. This suspension was filtered through a funnel lined with 2–3 layers of gauze into a 15 ml tube. The tube was then centrifuged at 1,500 rpm for 2 min to sediment the helminth eggs. The supernatant was discarded, and the sediment was resuspended in water. This centrifugation process was repeated 3–5 times until the supernatant became clear. After the final centrifugation, the supernatant was discarded, and 10 ml of 10% formalin was added to the tube to mix with the sediment and fix the helminth eggs for 5 min. Then, 3 ml of ether was added, and the mixture was vigorously shaken for 1 min before being centrifuged at 3,000 rpm again for 5 min. The upper debris layer was carefully removed with a wooden applicator along with the supernatant. The remaining sediment was resuspended and examined microscopically. During optical microscopy, the initial observation of helminth eggs was conducted at ×100 magnification, followed by a closer examination at ×400 magnification to assess the size and morphology. The size of the detected helminth eggs was measured using an ocular micrometer.
DNA was extracted from 101 fecal samples using the QIAamp Fast DNA Stool Mini Kit (Qiagen, Hilden, Germany) following the manufacturer’s instructions. DNA concentration and purity were measured using a NanoDrop One spectrophotometer (Thermo Scientific, Waltham, MA, USA), and the DNA was stored at −80°C until testing. The DNA extracted from 101 fecal samples was analyzed for protozoan infections using nested-PCR. In this method, the first round of PCR amplification was performed using outer primers, followed by a second round with an inner primer set that binds within the previously amplified region, enhancing specificity and sensitivity. Different primers and PCR conditions were applied for each of the 5 protozoan species: Blastocystis spp., Cryptosporidium felis, Enterocytozoon bieneusi, Giardia sp., and T. gondii (Supplementary Table S1). The second-round PCR products were run on a 1.5% gel for 30 min, and the presence of DNA amplification bands at expected positions was confirmed using a ChemiDoc Imaging System (Bio-Rad, Hayward, CA, USA). Samples with bands at expected positions underwent Sanger sequencing at Macrogen (Seoul, Korea) for final confirmation. Serum was separated from 237 blood samples and tested for T. gondii-specific antibodies (IgM and IgG) using an ELISA. To separate serum, blood collected in serum-separating tubes was centrifuged at 3,000 rpm for 15 min. The serum was stored in 1.5 ml tubes at −20°C until testing. The ID Screen Toxoplasmosis Indirect MultiSpecies Kit (IDvet, Montpellier, France) was used according to the manufacturer’s instructions. The optical density (OD) of the samples was corrected by subtracting the OD of the negative control, and results were normalized by dividing by the OD of the positive control. Samples were considered negative if the corrected OD was below the threshold and positive if it met or exceeded the specified cutoff value.
The positive rates of intestinal helminths, protozoa, and T. gondii-specific antibodies in stray cats were analyzed using the Chi-square test.
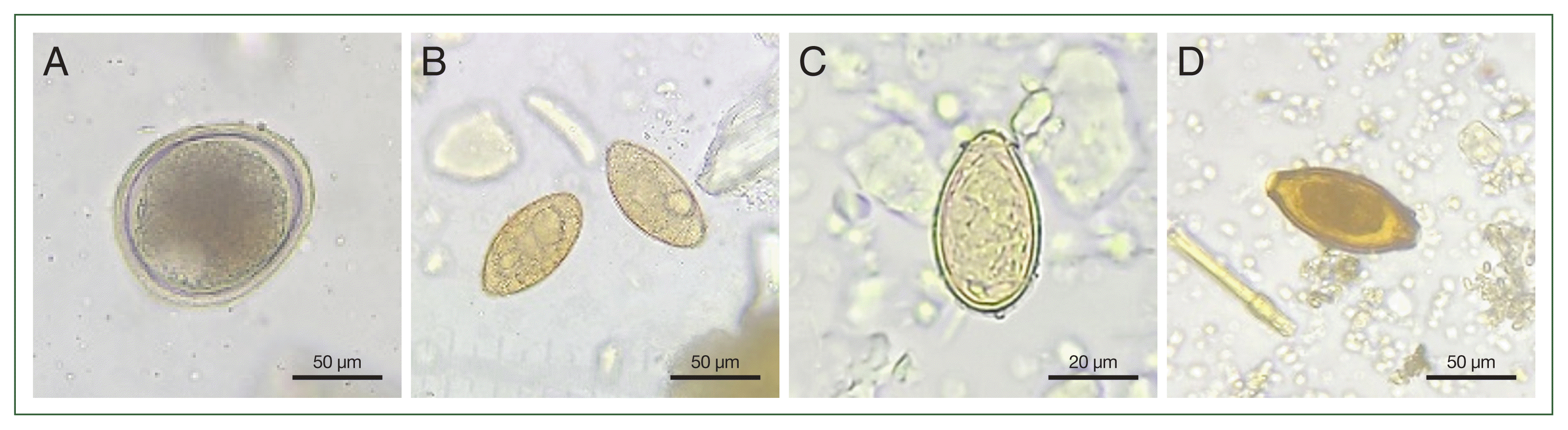
A total of 30 fecal samples (29.7%) tested positive for 4 species of helminth eggs: T. cati, Spirometra sp., Clonorchis sinensis, and Trichuris sp. (Table 1; Fig. 1). Among these, T. cati had the highest positive rate at 26.7% (27/101). Spirometra eggs were found in 2% (2/101), while C. sinensis and Trichuris eggs were each detected in 1% (1/101). Out of 101 fecal samples analyzed using nested-PCR and confirmed by Sanger sequencing, 16.8% (17/101) were tested positive for T. gondii, while C. felis and E. bieneusi were each detected in 4% (4/101) (Table 1). E. bieneusi-positive samples were distributed across different regions. In contrast, Giardia lamblia and Blastocystis spp. were not detected by molecular methods.
A total of 237 serum samples were tested for T. gondii-specific antibodies. The results showed that 21.9% (52/237) tested positive for IgM, 21.1% (50/237) tested positive for IgG, and 28.7% (68/237) tested positive for either IgM or IgG (Table 1). Statistical analysis showed no significant difference in T. gondii-specific antibody results based on sex and weight (data not shown). However, when analyzing the geographical distribution of T. gondii-infected stray cats, a high prevalence was observed in a specific region (Gochone-eup).
In this study, we investigated the prevalence of intestinal helminths and protozoan parasites in stray cats from Gimpo-si. Fecal analysis identified 4 species of helminth eggs: T. cati, Spirometra sp., C. sinensis, and Trichuris sp. (Fig. 1; Table 1). Additionally, molecular and serological analyses provided significant insights into the prevalence of protozoan parasites, specifically T. gondii, C. felis, and E. bieneusi in stray cats (Table 1).
T. cati is a common zoonotic parasite that can cause eosinophilia and various syndromes in humans [4]. Infection occurs through the accidental ingestion of infectious eggs from the environment or the consumption of undercooked meat from infected intermediate hosts [4]. This study confirmed that the positive rate of T. cati at 26.7% (27/101) was comparable to a previous survey result, which was 25.1% (102/407) in Daegu [3]. The epidemiological features of T. cati show that the higher prevalence in stray cats (28.6%) compared to household cats (11.6%) [4]. Given these findings, continuous control measures are necessary to mitigate potential health risks and prevent further transmission, particularly among stray cat populations.
Among intestinal helminths, Spirometra species found in cats pose significant public health concerns. Human sparganosis primarily occurs through the consumption of raw or undercooked meat from second intermediate hosts or by drinking untreated water contaminated with infected copepods [5]. Since cats and dogs serve as definitive hosts for Spirometra [5], ongoing monitoring and management strategies for Spirometra infection in natural hosts are crucial. Identifying Spirometra eggs based solely on morphology is challenging; therefore, DNA-based analysis is essential for accurate taxonomic classification [6]. In this study, the exact species of Spirometra could not be determined but is likely Spirometra decipiens or Spirometra erinaceieuropaei, both of which have been genetically identified in domestic cats in Korea [6].
Trichuris sp. is a common nematode in cats in Korea with zoonotic potential, meaning it can impact human health through environmental contamination or direct contact. Infections caused by Trichuris sp. are often asymptomatic or subclinical, with cats typically acquiring the infection through contaminated food or water. In this study, the positive rate was found to be 1%, which is lower than the 4.3% reported in a 2012 survey conducted in Suwon [7]. However, continued surveillance of stray cat populations is essential to prevent the re-emergence of trichuriasis. Unlike T. cati, C. sinensis has humans as its definitive host, with cats acting as reservoir hosts that contribute to environmental spread [8]. Infection with C. sinensis occurs through the consumption of raw freshwater fish containing C. sinensis metacercariae [9]. The C. sinensis positive-stray cat in this study was captured approximately 2 km from a tributary of the Han River, suggesting that the cat may have consumed infected fish, providing a potential route of transmission. Our study, along with previous research conducted across 26 regions nationwide, identified a C. sinensis positivity rate of 1% in stray cats [10]. Given these findings, it is crucial to adopt a One Health approach and implement management strategies to address the role of stray cats as reservoir hosts. T. gondii is a protozoan parasite capable of infecting most warm-blooded animals, including humans, with cats serving as the definitive hosts. Human infection with T. gondii can occur through multiple routes, including ingestion of contaminated food or water, contact with infected soil or cat feces, blood transfusion, organ transplantation, or vertical transmission from mother to fetus [11]. Infected humans can show a range of clinical manifestations, from asymptomatic cases to severe central nervous system involvement [11]. In 2008, the seropositive rate for T. gondii was 15.8% in stray cats from Seoul [12]. A nationwide study conducted between 2017 and 2019 found that the seroprevalence of T. gondii in stray cats was approximately 6 times higher than in household cats [13]. In this study, serological analysis revealed that a certain percentage of T. gondii showed that 28.7% (68/237) tested positive for either IgM or IgG. Notably, the prevalence rate in Gochon-eup was 91.7% (11/12), highlighting a distinct geographical distribution of T. gondii infections.
Our molecular analysis also provided significant insights into the prevalence of other protozoan parasites, specifically C. felis and E. bieneusi. A previous study on shelter cats from Jeju Island detected C. felis in 0.6% (1/158) of samples, while E. bieneusi was found in 3.8% (6/158) of cases [14]. In comparison, our study on stray cats demonstrated a higher prevalence of C. felis (4%) and a comparable prevalence of E. bieneusi (4%), suggesting potential regional variations in infection rates.
This study was conducted on cats captured for neutering surgery, which presents a limitation, as the results may not fully represent the overall infection status of cats in Gimpo-si. However, this research provides valuable insights into parasitic infections in stray cats within the metropolitan area, consistent with previous findings from Suwon [7]. Another limitation is that the formalin-ether method used in this study is particularly effective for detecting parasite eggs with higher specific gravity. Consequently, eggs of parasites with varying sizes and densities, such as hookworms, Strongyloides stercoralis, Fasciola sp., and Cytoisospora sp., which have lower specific gravity, may have been underestimated in terms of true infection prevalence [15]. Future studies should employ alternative diagnostic techniques, such as more sensitive flotation methods or molecular tools, to improve accuracy in detecting infections.
In this study, we confirmed a high prevalence of zoonotic parasite infections in stray cats from Gimpo-si, near Seoul. These findings provide comprehensive data on parasitic infections in stray cats and offer essential insights for the prevention and management of zoonotic parasitic diseases. Future research should expand to include diverse regions and both stray and pet animals to further investigate zoonotic parasitic infections.