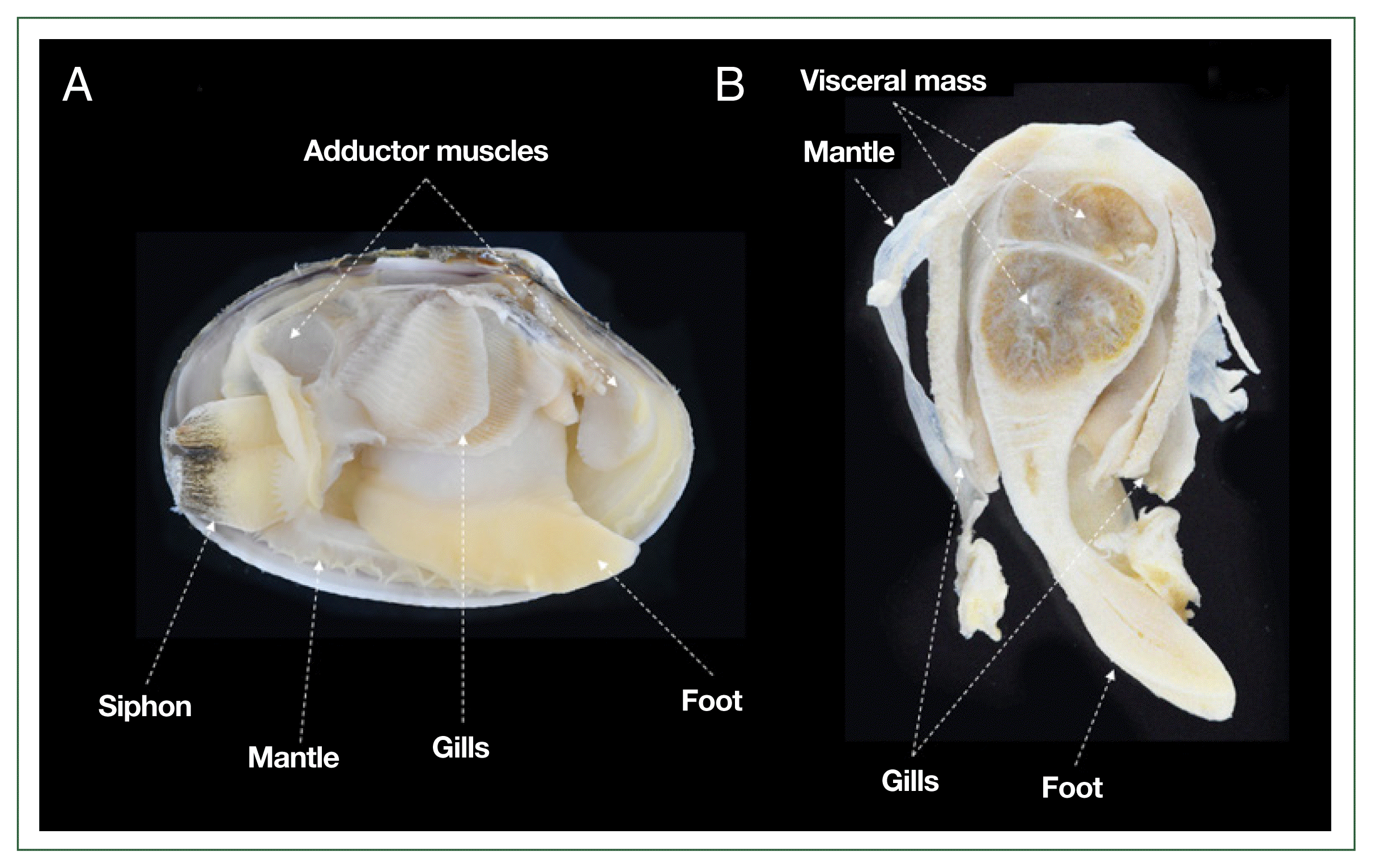
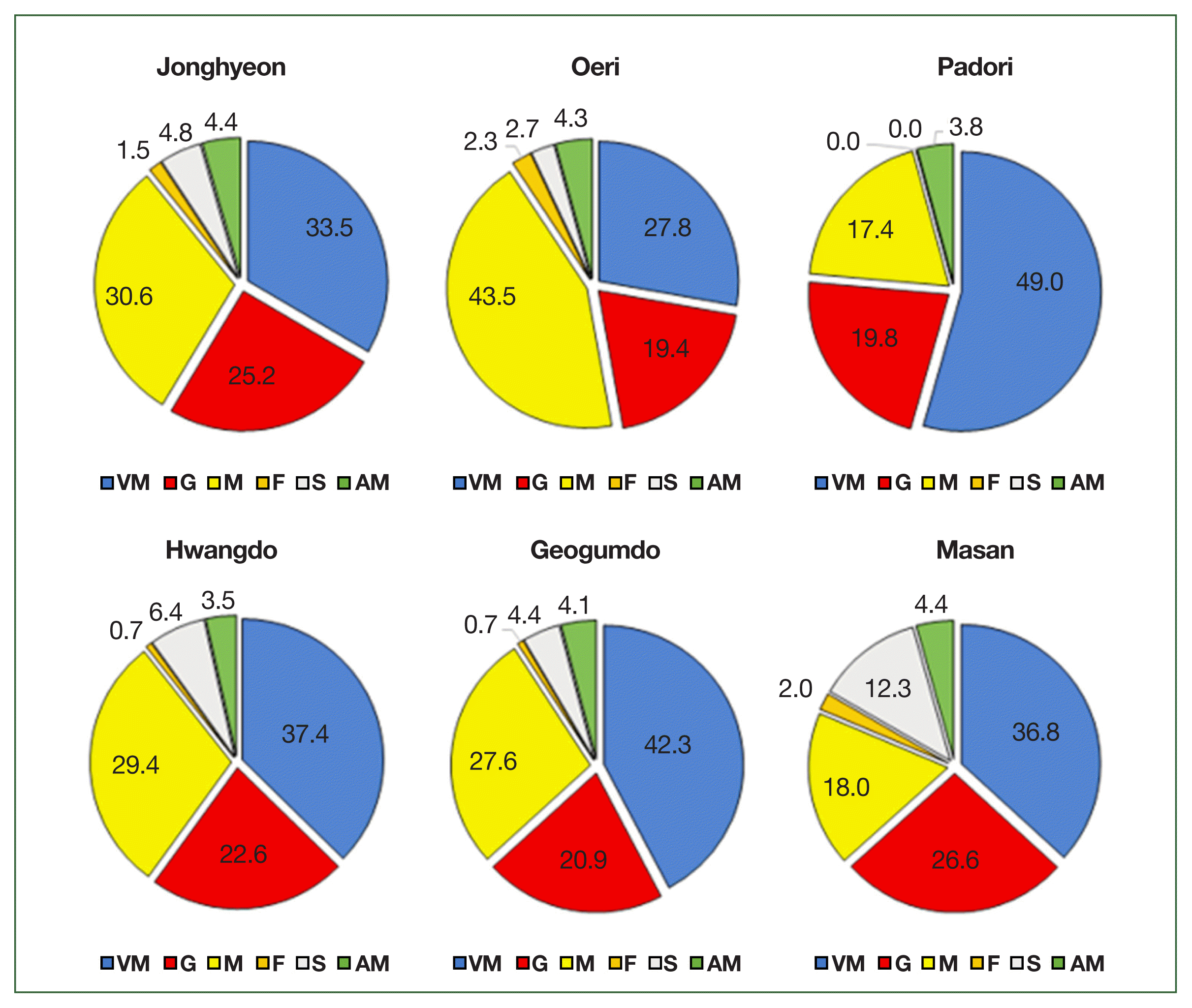
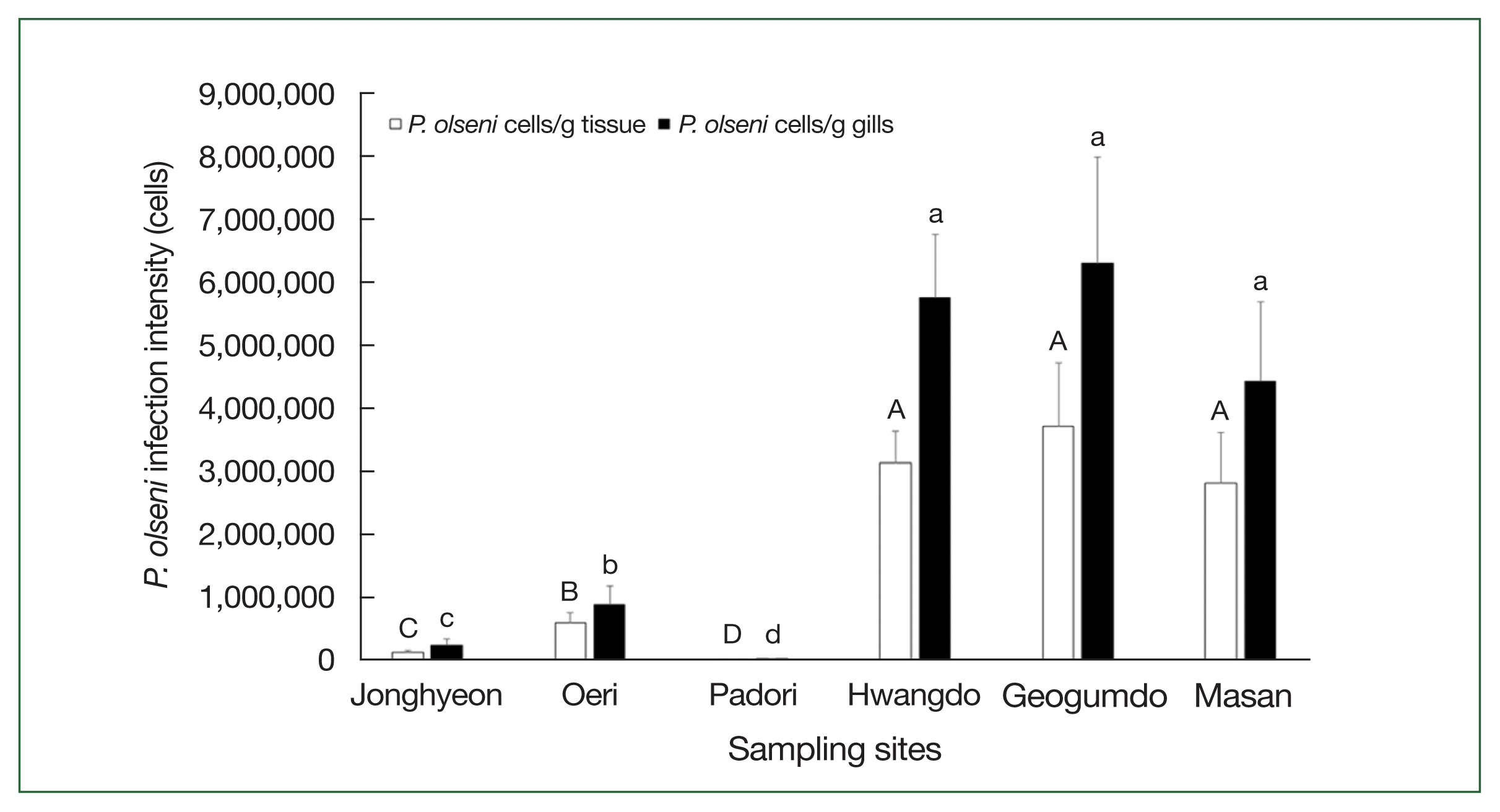
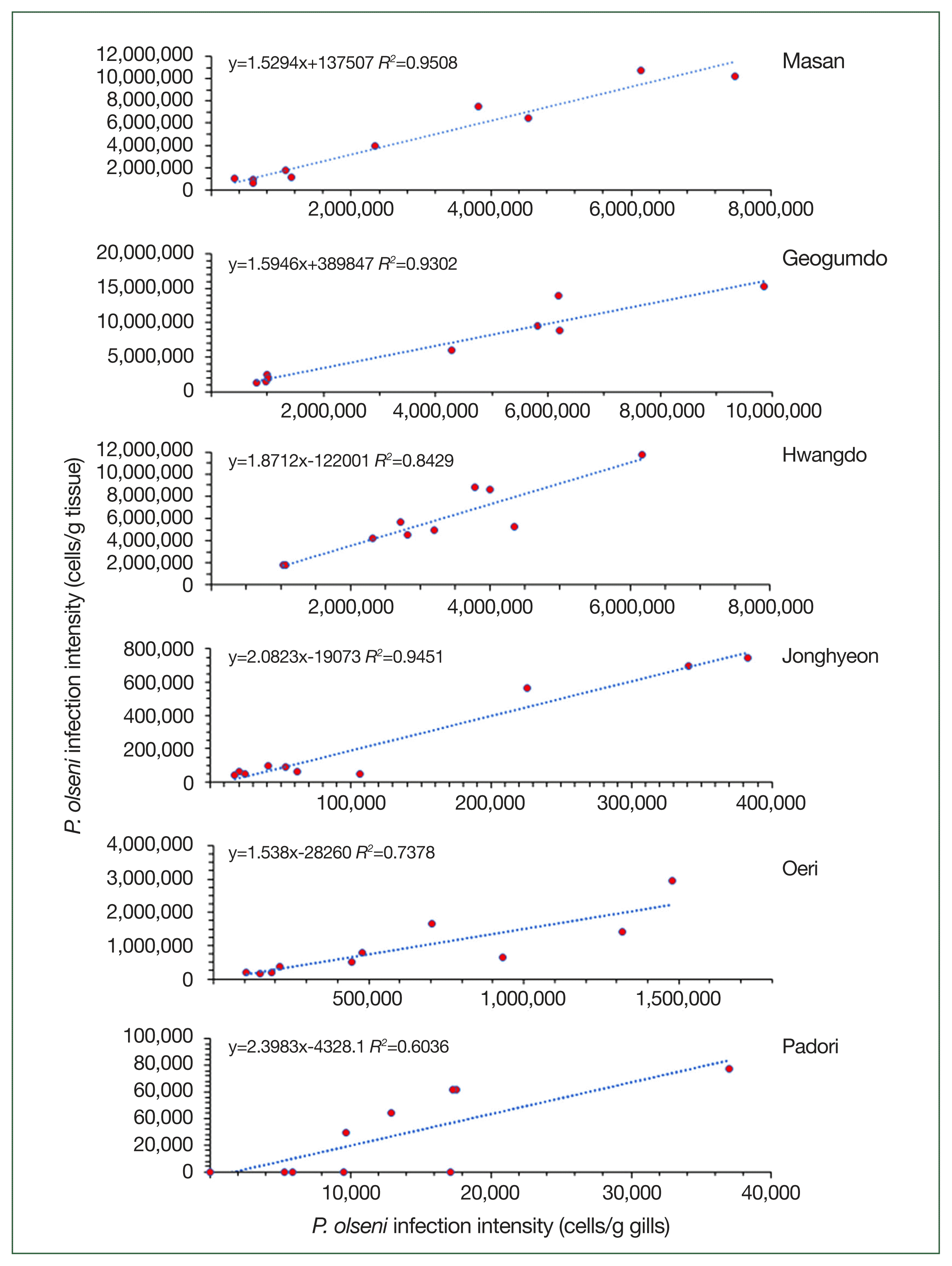
1. Costello KE, Lynch SA, O’Riordan RM, McAllen R, Culloty SC. The importance of marine bivalves in invasive host-parasite introductions. Front Mar Sci 2021;8:609248
https://doi.org/10.3389/fmars.2021.609248

3. Lynch SA, Rowley AF, Longshaw M, Malham SK, Culloty SC. Diseases of molluscs. In Rowley AF, Coates CJ, Whitten MM eds, Invertebrate Pathology. Oxford University Press. Oxford, UK. 2022, pp 171-215.

4. Park KI, Park JK, Lee J, Choi KS. Use of molecular markers for species identification of Korean
Perkinsus sp. isolated from Manila clams
Ruditapes philippinarum. Dis Aquat Org 2005;66(3):255-263
https://doi.org/10.3354/dao066255


5. Limpanont Y, Kang HS, Hong HK, Jeung HD, Kim BK, et al. Molecular and histological identification of
Marteilioides infection in Suminoe oyster
Crassostrea ariakensis, Manila clam
Ruditapes philippinarum and Pacific oyster Crassostrea gigas on the south coast of Korea. J Invertebr Pathol 2013;114(3):277-284
https://doi.org/10.1016/j.jip.2013.08.008


6. Itoh N, Yamamoto T, Kang HS, Choi KS, Green TJ, et al. A novel paramyxean parasite,
Marteilia granula sp. nov. (Cercozoa), from the digestive gland of Manila clam
Ruditapes philippinarum in Japan. Fish Pathol 2014;49(4):181-193
https://doi.org/10.3147/jsfp.49.181

7. Kang HS, Itoh N, Limpanont Y, Lee HM, Whang I, et al. A novel paramyxean parasite,
Marteilia tapetis sp. nov. (Cercozoa) infecting the digestive gland of Manila clam
Ruditapes philippinarum from the southeast coast of Korea. J Invertebr Pathol 2019;163:86-93
https://doi.org/10.1016/j.jip.2019.03.006


8. Cho YG, Kang HS, Le CT, Kwon MG, Jang MS, et al. Molecular characterization of
Urosporidium tapetis sp. nov., a haplosporidian hyperparasite infecting metacercariae of
Parvatrema duboisi (Dollfus 1923), a trematode parasite of Manila clam
Ruditapes philippinarum on the west coast of Korea. J Invertebr Pathol 2020;175:107454
https://doi.org/10.1016/j.jip.2020.107454


9. Le CT, Jeung HD, Cho YG, Choi KS. Survey of trematodes in Manila clam
Ruditapes philippinarum on the west coast of Korea: a preliminary study. J Invertebr Pathol 2024;206:108172
https://doi.org/10.1016/j.jip.2024.108172


10. Park KI, Figueras A, Choi KS. Application of enzyme-linked immunosorbent assay (ELISA) for the study of reproduction in the Manila clam
Ruditapes philippinarum (Mollusca: Bivalvia): II. Impacts of
Perkinsus olseni on clam reproduction. Aquaculture 2006;251(2–4):182-191
https://doi.org/10.1016/j.aquaculture.2005.06.003

12. Lee HM, Cho YG, Jeung HD, Jang MS, Hwang JY, et al. Are juvenile Manila clam
Ruditapes philippinarum free from
Perkinsus olseni infection in Korean waters? Ocean Sci J 2020;55:573-579
https://doi.org/10.1007/s12601-020-0038-2

13. Lee HM, Park KI, Yang HS, Choi KS. Negative impacts of
Perkinsus olseni infection in Manila clam
Ruditapes philippinarum observed from tidal flats in Anmyeondo Island on the west coast of Korea during post-spawning period. Ocean Sci J 2021;56:307-316
https://doi.org/10.1007/s12601-021-00024-0

14. Yang HS, Park KI, Donaghy L, Adhya M, Choi KS. Temporal variation of
Perkinsus olseni infection intensity in the Manila clam
Ruditapes philippinarum in Gomso Bay, off the west coast of Korea. J Shellfish Res 2012;31(3):685-690
https://doi.org/10.2983/035.031.0312

15. Choi KS, Park KI, Lee KW, Matsuoka K. Infection intensity, prevalence and histopathology of Perkinsus sp. in the Manila clam, Ruditapes philippinarum, in Isahaya Bay, Japan. J Shellfish Res 2002;21:119-125.
16. Uddin MJ, Yang HS, Choi KS, Kim HJ, Hong JS, et al. Seasonal changes in
Perkinsus olseni infection and gametogenesis in Manila clam,
Ruditapes philippinarum, from Seonjaedo Island in Incheon, off the west coast of Korea. J World Aquac Soc 2010;41:93-101
https://doi.org/10.1111/j.1749-7345.2009.00337.x

17. Kang HS, Yang HS, Reece KS, Cho YG, Lee HM, et al. Survey on
Perkinsus species in Manila clam
Ruditapes philippinarum in Korean waters using species-specific PCR. Fish Pathol 2017;52(4):202-205
https://doi.org/10.3147/jsfp.52.202

19. Dungan CF, Bushek D. Development and applications of Ray’s fluid thioglycollate media for detection and manipulation of
Perkinsus spp. pathogens of marine molluscs. J Invertebr Pathol 2015;131:68-82
https://doi.org/10.1016/j.jip.2015.05.004


21. Choi KS, Wilson EA, Lewis DH, Powell EN, Ray SM. The energetic cost of Perkinsus marinus parasitism in oysters: quantification of the thioglycollate method. J Shellfish Res 1989;8:125-131.
22. Leethochavalit S, Chalermwat K, Upatham ES, Choi KS, Sawangwong P, et al. Occurrence of
Perkinsus sp. in undulated surf clams
Paphia undulata from the Gulf of Thailand. Dis Aquat Org 2004;60(2):165-171
https://doi.org/10.3354/dao060165


23. Dang C, de Montaudouin X, Binias C, Salvo F, Caill-Milly N, et al. Correlation between perkinsosis and growth in clams
Ruditapes spp. Dis Aquat Org 2013;106(3):255-265
https://doi.org/10.3354/dao02640


24. Yang HS, Cho YG, Shin JS, Park HS, Choi KS. Pathology survey of the Manila clam
Ruditapes philippinarum from Hwangdo tidal flat in Cheonsu Bay on the west coast of Korea. Ocean Polar Res 2021;43(4):365-370
https://doi.org/10.4217/OPR.2021.43.4.365

25. Subramaniam T, Cho YG, Lee HM, Kim JH, Shin JS, et al. Spatio-temporal variation in
Perkinsus olseni infection intensity in Manila clam
Ruditapes philippinarum in Anmyeondo and Cheonsu bay tidal flats on the west coast of Korea. Ocean Sci J 2024;59(1):1-10
https://doi.org/10.1007/s12601-023-00125-y