Abstract
Microphallus species occur primarily as intestinal parasites of birds and mammals, and metacercariae of a new species belonging to this genus have been discovered from the crab, Macrophthalmus dilatatus, in the Republic of Korea. The metacercaria of this fluke was round with 2 thick walls, and the excysted one had mature genital organs. The adult flukes recovered from experimentally infected chicks had numerous intrauterine eggs, well-developed pars prostatica, widely bifurcating ceca, and prominent uterine bulge. After observing internal structures, it was concluded that this species is different from any other known Microphallus spp. Based on the morphology of metacercariae and adult flukes, we describe this specimen as a new species, Microphallus koreana n. sp.
-
Key words: Microphallus koreana, microphallid, Macrophathalmus dilatatus, crab, chick
INTRODUCTION
After the first larval microphallid was described by McIntosh [
1] in Scotland from the marine crabs, Ward [
2] created the genus
Microphallus, and Cable and Hunninen [
3] described the experimental life cycle for the genus
Microphallus with
Microphallus nicolli. Like
Microphallus primas which had been found in the oystercatcher, the adult flukes of the family Microphallidae Travassos, 1920 occur primarily as intestinal parasites of birds [
4]. Sexually mature adults of
M. primas had been recorded in the oystercatcher and eider duck [
5], and the definitive hosts of
Microphallus papillorubustus are various aquatic birds. According to Fauna Europaea [
6], the genus
Microphallus Ward 1901 was proved to be identical with
Bulbovitellus Yamaguti 1971,
Spelophallus Jagerskiold 1908, and also with
Spelotrema Jagerskiold 1901. Total 18 species of
Microphallus have been recorded until now, after Deblock and Maillard [
7] described
Microphallus breviatus as a new species, and Diaz et al. [
8] added a new species,
Microphallus sabanensis, from Venezuela.
The metacercarieae of this trematode are usually found in crustaceans [
9]. For example, the grass shrimp is the second intermediate host for
Microphallus turgidus [
10], and the crayfish for
Microphallus opacus [
4]. The second intermediate host of
M. sabanensis was a crab in Venezuela [
8]. The Republic of Korea has a lot of tidal flats along the western shoreline, where numerous crustaceans dwell inside, but there has been no report on the presence of
Microphallus spp. yet. We found the metacercariae of
Microphallus sp. in the crab,
Macrophthalmus dilatatus, and obtained its adult flukes after experimental infection to chicks. By morphologically differentiating them with pre-existing worms of
Microphallus spp., we propose our worms as a new species of the genus
Microphallus.
MATERIALS AND METHODS
Isolation of metacercariae
One kilogram of marine crabs, M. dilatatus, were purchased at a fish market in Taean-eup, Chungcheongnam-do, Republic of Korea, in June 2007. Considering that the average weight of the crab was 4.3 g, the number of crabs used in this experiment was estimated to be 232. The crabs were crushed and Microphallus sp. metacercariae were collected under a stereomicroscope after several washings with 0.85% saline. The collected metacercariae were used for experimental infection, and some of them were excysted and fixed in 10% neutral formalin for microscopic examinations.
Experimental animals and infection
Specific-pathogen free (SPF) Sprague-Dawley (SD) rat (5-wk-old) were purchased from the Koatech Co., Ltd. (Pyeongtaek, Korea) and maintained under SPF condition at the animal facility of Dankook University. The chicks (3-day-old) were obtained from the Hanil Hatchery Co., Ltd (Osan, Korea) and maintained as above. To obtain adult flukes, 5 rats were orally fed 100 metacercariae each, and 3 chicks 200 metacercariae each. They were killed at day 5 day post-infection (PI) referring to the report of Galaktionov and Skirnisson [
11], and small intestines were removed. To know the habitat of adult flukes, the small intestine was divided into 3 portions and longitudinally resected. The worms were recovered from the intestinal contents under a stereomicroscope, and their number was counted. The collected flukes were fixed in 10% neutral formalin and stained with Semichon's acetocarmine. They were observed under light microscopy, and their morphology was described. Drawing of the worm was done.
DESCRIPTION
Microphallus koreana n. sp.
Metacercaria
The collected metacercariae of
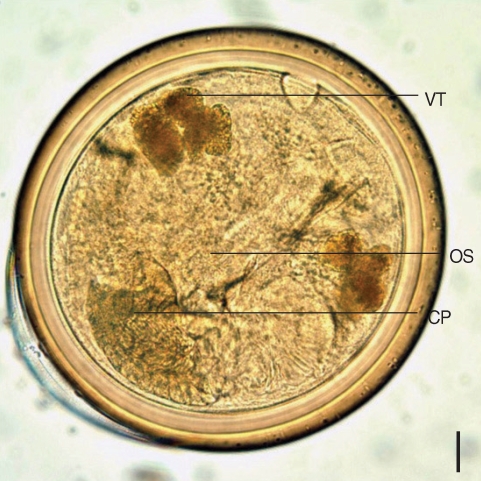
Microphallus sp. was slightly over 1,100 in number, and the mean number of metacercariae per crab was 4.7. The metacercariae were mainly located in the hepatopancreas of the crab. Morphological characters of the metacercariae were as follows; cyst (
Fig. 1) round, 320 µm in diameter with 2 thick walls, which composed of an outer layer 12.5 µm and an inner layer 15.7 µm. Oral sucker is clearly visible, and the muscular pharynx is close to the oral sucker. Vitelline glands are well seen, and the seminal vesicle prominent.
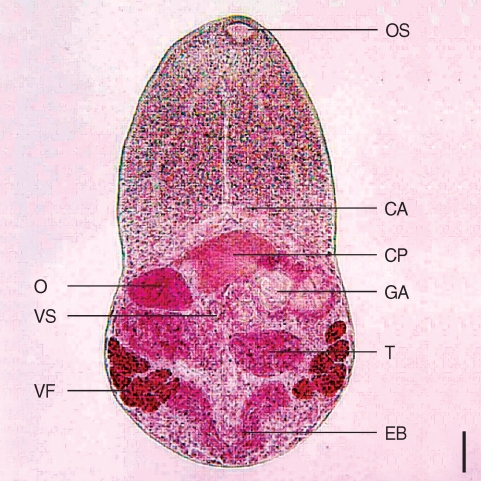
The detailed morphology could be seen in the excysted metacercariae (
Fig. 2). It looked similar to adults except for the presence of intrauterine eggs. Body slender, slightly constricted at posterior two-third of the body and 545.0 × 308.0 µm in average size. Anterior end streamlined, but posterior part somewhat wider with a round end. Oral sucker terminal, and prepharynx invisible. Pharynx muscular, esophagus long, bifurcating into 2 intestinal ceca in front of cirrus pouch. Cirrus pouch consisted of par prostatica and seminal vesicle, located just posterior to cecal arch. The former was well-developed, but the latter was invisible. Ovary located beneath the end of right intestinal cecum. Ventral sucker very small, nearly one-forth of the oral sucker, left to the ovary. Right testis posterior to ovary, slightly anterior to the left one. Genital atrium posterosinistral to seminal vesicle, and genital pore inside it. Uterine tube had a bulge near end of the left cecum. Vitelline glands arranged in 2 groups, 7 follicles on the right and 6 on the left.
Adult
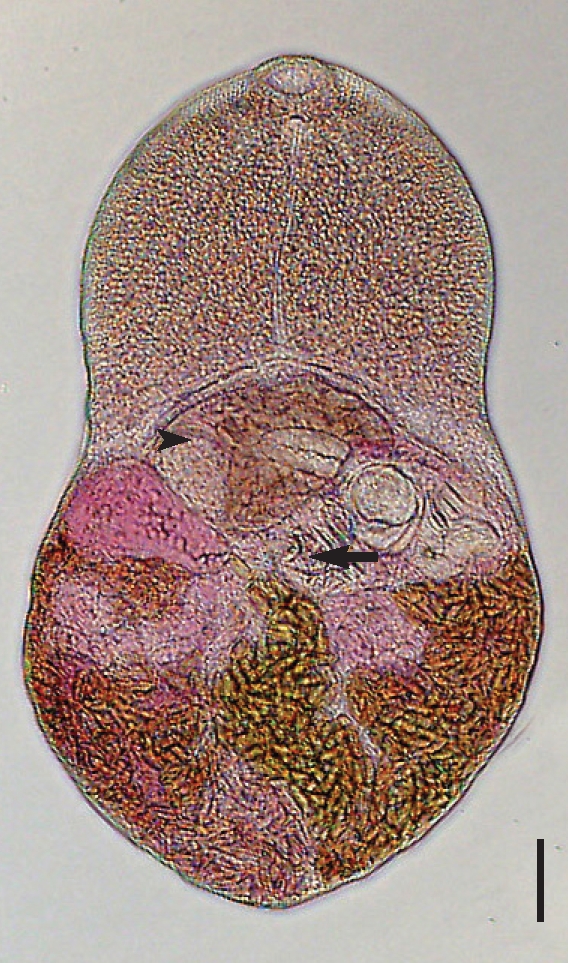
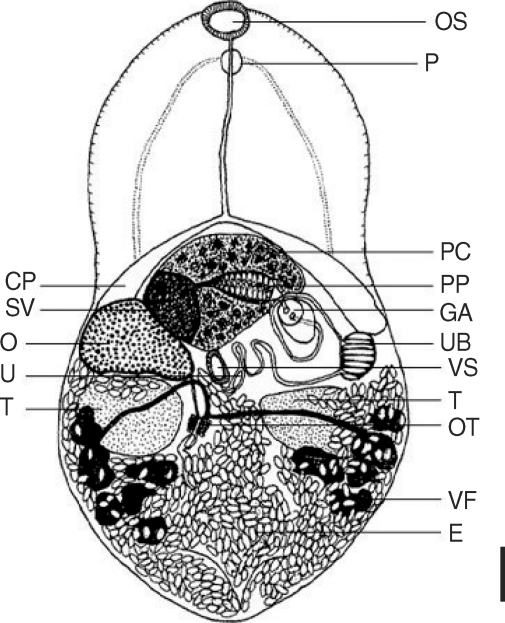
While no worms were recovered from 5 rats, total 12 adults of
Microphallus sp. were recovered from 3 chicks (mean worm recovery rate, 2%). All the worms were recovered from the ileum. Description based on 12 adult flukes is as follows (
Fig. 3): Body bilobed, resembling the rice scoop, 572.5 (530-560) µm long and 372.5 (330-400) µm in maximum width at testicular level. Tegumental spines distributed from oral sucker to the level of intestinal ceca. Oral sucker terminal, 40.8 (35-50) × 62.8 (50-70) µm in size. Prepharynx 10.6 (5-25) µm, esophagus 138.8 (100-163) µm. Ventral sucker small, 37.8 (38-40) × 33.4 (33-38) µm. Sucker ratio 1 : 0.49. Cirrus pouch large, having seminal vesicle and pars prostatica inside. Seminal vesicle well-developed, 69.8 (63-73) × 63.8 (50-78) µm, posterior to cecal arch. Pars prostatica prominent with abundant prostate cells. Uterus occupied post-testicular region, having numerous eggs. Uterine bulge was prominent at the end of left cecum (
Fig. 5). Ovary 66.4 (55-73) × 106.1 (55-63) µm, posterior to the right ceca. Seminal receptacle not observed. Right testis 61.6 (55-63) × 104.8 (88-113) µm and left one 61.6 (50-75) × 108.2 (80-125) µm. Sinistral to right testis, ootype could be seen. Vitelline duct undulating but nearly transverse at testicular level. The post-testicular region filled with numerous intrauterine eggs, making the vitelline follicle indistinguishable. Intrauterine eggs elliptical (
Fig. 4), 22.5 × 12.5 µm in size with inconspicuous operculum. Excretory bladder invisible due to eggs.
Experimental host: Chick.
Site of infection: Small intestine, posterior portion.
Second intermediate host: Crab, Macrophthalmus dilatatus.
Localities: Taean-gun, Chungcheongnam-do (Province), the Republic of Korea.
Etymology: Species name means the Republic of Korea.
Holotype: Adult fluke is deposited in Department of Parasitology, College of Medicine, Dankook University, Cheonansi, Chungnam, 330-714, the Republic of Korea.
DISCUSSION
Following the classification of Yamaguti [
12], the species described above, namely
M. koreana, fits the criteria of the genus
Microphallus: well-developed pars prostatica, widely bifurcating ceca, and prominent uterine bulge. However,
M. koreana had several unique characteristics differed from other species of
Microphallus (
Table 1) [
8,
11-
13]. Briefly,
M. koreana is smaller in length than
Microphallus longicolle, but longer than
M. breviatus,
M. papillorobustus,
Microphallus triangulates,
Microphallus pseudophagmaeus, and
Microphallus pirum. It is wider than
Microphallus fusiformis by 6 times. The eggs of
M. koreana are smaller than those of
M. triangulatus,
M. pseudophagmaeus,
M. pirum, and
M. fusiformis. The adult worms have numerous intrauterine eggs unlike
M. pirum and
M. fusiformis. The oral sucker of
M. koreana is bigger than the ventral sucker, while
Microphallus claviformis,
M. sabanensis,
M. primas, and
Microphallus limuli had the ventral suckers of a same size with their oral suckers. These findings are enough to suggest the present worms as a new species.
Ching [
14] suggested that microphallid species are known for rapid attainment of sexual maturity in the intestine of the definitive host. The velocity of maturity, however, varied by species, and
Microphallus similis matured after 4 days when mice were used as an experimental host [
15]. As for
Microphallus pygmaeus, the maximum number of eggs was attained by day 8 PI in laboratory mice [
16]. In the present study, the worm recovery was done at day 5 PI, but the recovery rate of
M. koreana was only 2% in chicks and 0% in mice. It is suggested that most adult worms had been expelled prior to that. Considering the 5-day old worms were full of intrauterine eggs,
M. koreana seemed to become maturity rapidly, and an earlier worm recovery should be tried to obtain more adult worms.
Caveny and Etges [
4] suggested that there was a high probability of the life history of
M. opacus being completed without involvement of a vertebrate host. It was supported by Saville and Irwin [
14], who succeeded in developing
M. primas metacercariae into adults in the laboratory. However,
M. sabanensis required bird or mammalian definitive hosts for completing its life cycle [
8], and Raush [
15] reported the adult worms of
M. opacus in mammalian hosts. Even though the experimental infection could be possible, the intestinal environment of mice seemed to be not suitable for
Microphallus sp. The metacercariae of
M. pygmaeus rapidly died in vitro in conditions that simulated the environment in the small intestine of mice [
19], and longevity of infection did not exceed 12 days even for the successfully settled worms [
20]. This could explain why no worm was recovered from the rats in the present study. In this regard, human infections by
M. koreana might be infrequent along with the fact that these kinds of crabs have not been consumed in raw state.
The rapid excystment of this species was worth to pay attention. The excystment pattern of
Micropahllus differed according to each species, and
M. primas metacercariae, for example, did not excyst spontaneously [
17]. While the metacercariae of
M. sabanensis did not excyst in normal saline for 6 hr [
8], those of
M. opacus excysted in vitro within 2 hr of removal from their host [
4]. In the present study, the collected metacercariae rapidly excysted in the laboratory condition, making the experimental infection to animals difficult. This phenomenon might explain that there had been no reports on this species in the Republic of Korea.
Unlike other trematodes, many
Microphallus species conduct dixenous life cycles [
12]. Namely, cercariae of these species do not leave the molluscan host but develop into metacercariae inside the daughter sporocysts, and such examples include
M. pirum,
Microphallus scolectroma,
Microphallus abortivus,
Microphallus simillimus, and
M. fusiformis. Since the second intermediate host of
M. koreana is not a molluscan but a crustacean, the presence of the first intermediate host is certain. Including the discovery of
M. koreana cercariae, more intention about this species should be tried.
References
Fig. 1A metacercaria of Microphallus koreana n. sp. isolated from the crab, Macrophthalmus dilatatus. OS, oral sucker; CP, cirrus pouch; VT, vitellaria. Bar = 30.8 µm.

Fig. 2An excysted metacercaria of M. koreana n. sp. showing the ovary, testes, seminal vesicle, and vitelline follicles. Bar = 43.6 µm. OS, oral sucker; CP, cirrus pouch; EB, excretory bladder; GA, genital atrium; O, ovary; VS, ventral sucker; T, testes; VF, vitelline follicle.

Fig. 3A 5-day-old worm of M. koreana n. sp. recovered from an experimental chick, stained with Semichon's acetocarmine. The ventral sucker (arrow) and the cirrus pouch with seminal vesicle (arrowhead) are recognized. Bar = 44.2 µm.

Fig. 4An egg of M. koreana n. sp. × 1,000. Bar = 4.2 µm.

Fig. 5Drawing of Microphallus koreana n. sp., an adult fluke recovered from the small intestine of an experimental chick at day 5 PI. OS, oral sucker; P, pharynx; PC, prostatic cells; CP, cirrus pouch; PP, pars prostatica; GA, genital atrium; UB, uterine bulge; U, uterus; SV, seminal vesicle; O, ovary; VS, ventral sucker; OT, oötype; T, testes; VF, vitelline follicle; E, egg. Bar = 44.2 µm.

Table 1.Comparison of the measurements of Microphallus koreana n. sp. with other Microphallus species (unit: μm)
Table 1.
|
Organs |
|
M. koreana
|
M. capellae
|
M. longicolle
|
M. similis
|
M. limuli
|
M. sabanensis
|
M. primas
|
|
Body length (L) |
|
530-630 |
400-510 |
720-940 |
360-700 |
260-400 |
390-555 |
775-835 |
|
Width (W) |
|
330-400 |
170-240 |
200-240 |
220-360 |
150-200 |
252-343 |
240-270 |
|
Oral sucker |
L |
35-50 |
34-45 |
54-80 |
50-60 |
35-45 |
45-65 |
52-54 |
|
W |
50-70 |
36-48 |
42-60 |
- |
- |
- |
- |
|
Prepharynx |
L |
5-25 |
20-50 |
60-150 |
- |
- |
- |
30-38 |
|
Pharynx |
L |
28-33 |
18-24 |
24-33 |
20-30 |
17-21 |
24-27 |
26-33 |
|
Esophagus |
L |
100-163 |
50-130 |
160-300 |
- |
100-130 |
59-123 |
230-300 |
|
Seminal |
L |
63-73 |
30-80 |
- |
- |
- |
- |
63-66 |
|
vesicle |
W |
50-78 |
- |
- |
- |
- |
80-100 |
|
|
Ventral |
L |
38-40 |
30-40 |
34-48 |
48-65 |
29-35 |
45-67 |
- |
|
sucker |
W |
33-38 |
|
|
|
|
|
|
|
Ovary |
L |
55-73 |
40-60 |
36-54 |
60-90 |
36-45 |
37-75 |
85-92 |
|
W |
33-113 |
50-75 |
48-63 |
100-160 |
60-65 |
53-86 |
92-108 |
|
Testis |
L |
55-63 |
- |
- |
100-160 |
50-65 |
43-80 |
54-64 |
|
W |
88-120 |
- |
- |
- |
- |
80-212 |
82-92 |
|
Eggs |
L |
20 |
18-24 |
21-24 |
22-27 |
16-20 |
18-21 |
- |
|
W |
13 |
9-11 |
12-15 |
11-12 |
9-11 |
8-10 |
- |