Abstract
We report here a case of oral myiasis in the Republic of Korea. The patient was a 37-year-old man with a 30-year history of Becker's muscular dystrophy. He was intubated due to dyspnea 8 days prior to admission to an intensive care unit (ICU). A few hours after the ICU admission, 43 fly larvae were found during suction of the oral cavity. All maggots were identified as the third instars of Lucilia sericata (Diptera: Calliphoridae) by morphology. We discussed on the characteristics of myiasis acquired in Korea, including the infection risk and predisposing factors.
-
Key words: Lucilia sericata, oral myiasis, predisposing factor, nosocomial infection
INTRODUCTION
Oral myiasis is regarded as a type of wound myiasis strongly associated with poor oral hygiene. Many other factors can also play a role, including alcoholism, senility, suppurating lesions, gingival diseases, trauma, paralysis or immobility, and mental debility [
1-
3].
The most common causative agent of myiasis, the dipteran clade Calyptratae, consists of approximately 18,000 described species, which represents approximately 12% of the known dipteran diversity. Four families of the Calyptratae, including Calliphoridae (blowflies), Sarcophagidae (flesh flies), Oestridae (bot flies), and Muscoidea (dung flies), have been implicated in causing most of the specific or facultative myiasis in humans [
4].
Among the members of the Calliphoridae,
Lucilia species are distributed throughout the world. They are considered important in the field of medical and veterinary science as causative agents of myiasis in humans and animals.
Lucilia sericata is commonly called as the green bottle blowfly or sheep strike blowfly. It is known as the predominant pathogenic species in cool temperate habitats such as New Zealand [
5].
The present paper reports a case of oral myiasis caused by L. sericata third instars larvae and documents the morphological characters of maggots observed by light and scanning electron microscopes (SEM). We also discuss the characteristics of 6 cases of myiasis acquired in Korea, including the risk of accidental myiasis in patients with predisposing factors.
CASE DESCRIPTION
Secondary to the diagnosis of Becker's muscular dystrophy, the patient (37-year-old man) has been bedbound for 30 years. On the evening of 21 October 2011, he complained of dyspnea and chest pain while at his nursing home. His blood pressure dropped to 60/30 mmHg and his mental status became drowsy. He was transferred to the emergency room (ER) of a university hospital, and emergency treatment was performed, including intubation. After spending 8 days in the ER, the patient was moved to an intensive care unit (ICU) in Busan St. Mary's Medical Center by the request of a guardian. The patient had symptoms, including hematochezia, fever, and chills. Laboratory findings included WBC of 5,200/mm3, hemoglobin (Hb) concentration of 12.5g/dl, and C-reactive protein (CRP) of 159.45 mg/L. Chest CT scans revealed bronchiectasis in the left lung field. There were no specific findings on abdominal CT scans. Several hours after transfer to the ICU, 38 maggots were removed during suction of the oral cavity. Two hours later, 5 more maggots were removed from the oral cavity. A fecal occult blood test was positive, and Hb taken on the next day was less than the normal range (11.6 g/dl). Laryngoscopic examination revealed no additional maggots or wounds in the nasal cavity or laryngopharyngeal space. The patient's condition improved and he returned to the nursing home on 26 December 2011.
For light microscopic observations, the larvae were preserved in 70% ethanol, and then they were cleared in a 10% aqueous solution of KOH. After clearing, the specimens were mounted on slides using glycerin-jelly medium. Additional maggots were preserved in 2% glutaraldehyde for SEM analysis. After washing 3 times with 2% glutaraldehyde, the specimens were dehydrated through a graded alcohol series (50%, 70%, 80%, 90%, 95%, and absolute alcohol), dried with hexamethyldisilazane, coated with gold (JFC-1100E ion sputtering device), and observed with Electron SEM Philips XL-30S (Philips, London, UK) at a 15-kV accelerating voltage. Species identification was done using the keys described by previous workers [
6,
7] and the Natural History Museum (London, UK) website (
http://www.nhm.ac.uk/research-curation/research/projects/myiasis-larvae/key/).
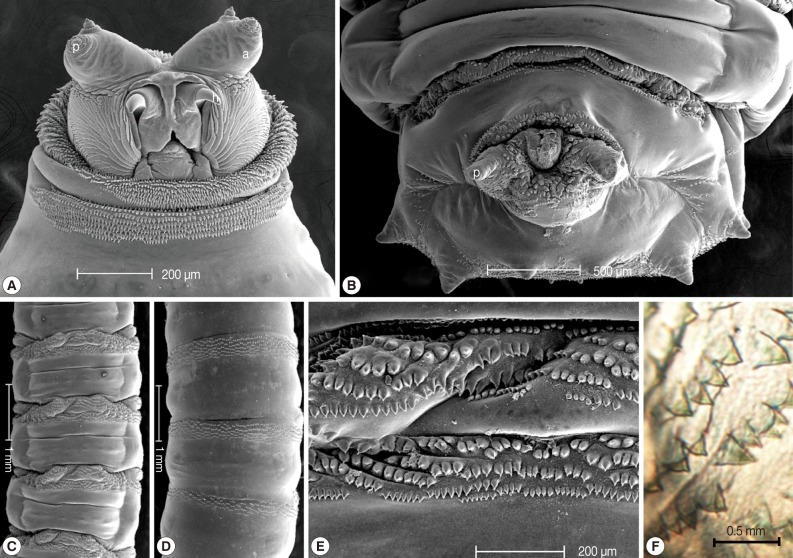
The collected maggots were 9-12 mm (mean; 10.3 mm) in length with a beige coloring (
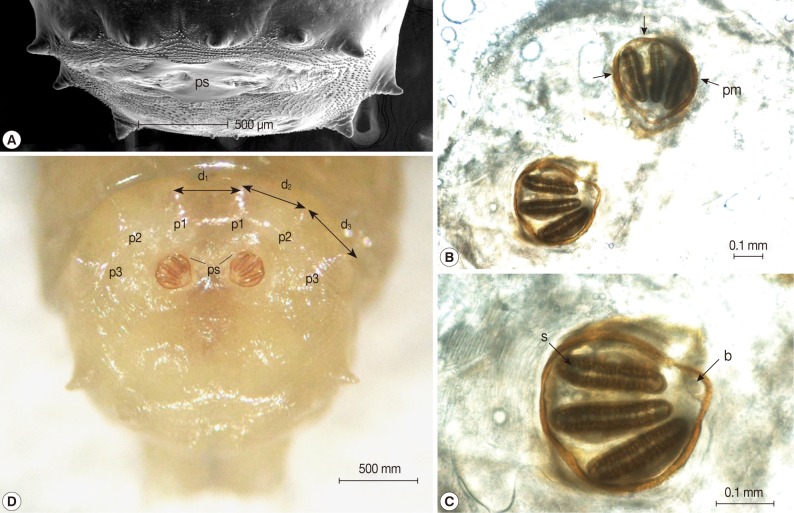
Fig. 1). The larvae showed the typical maggot-like body shape. The pseudocephalon had a pair of blunting antennae, each equipped with 2 sensory papillae. A pair of hooks was also located in the buccal cavity (
Fig. 2A). A pigmented accessory oral sclerite was not observed in the cephalopharyngeal skeleton. The anus had 1 pair of anal papillae surrounded by numerous tiny spines and was located under the posterior spiracular plate in the last segment (
Fig. 2B). The body surface was covered with many tiny short spines. The body spines were sharp with a single point and arranged in distinct bands around the body showing clearly defined spine bands (
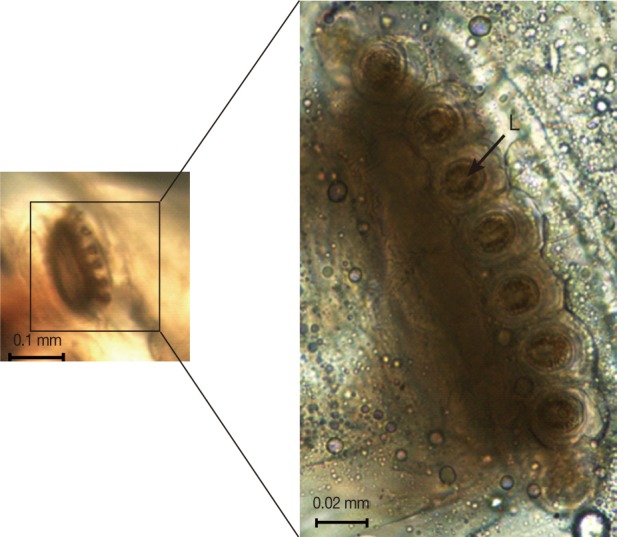
Fig. 2C-F). Anterior spiracles showed a fan-like structure with 8 lobes (
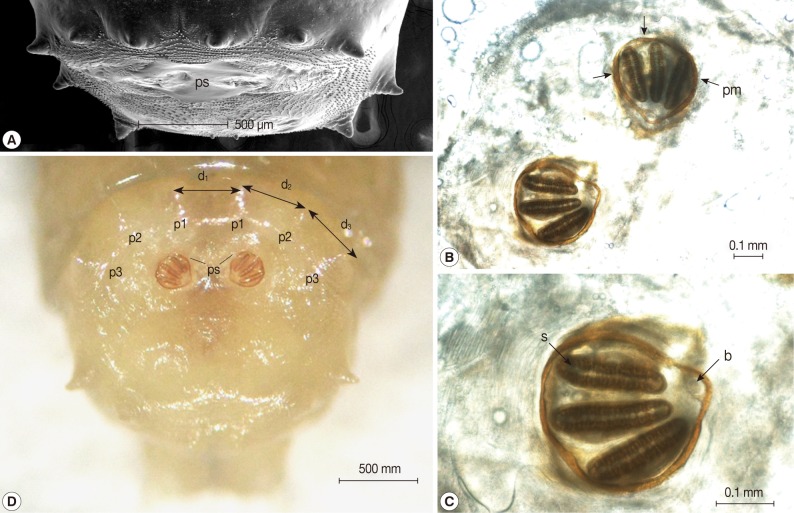
Fig. 3). The posterior spiracles were not positioned within a cavity (
Fig. 4A). The shape of the posterior spiracles was approximately round with a surrounding complete peritremal ring with no gap (
Fig. 4B). The button was present on both spiracles, and 3 slits in each posterior spiracle were straight (
Fig. 4C). The distances (d
1, d
2, and d
3) of the dorsal papillae (p1), median papillae (p2), and outer papillae (p3) in the 12th segment were similar (
Fig. 4D).
DISCUSSION
The family Calliphoridae contains more than 1,100 species, which can be found in almost every terrestrial habitat. Most of the species are of medical importance due to their abundance and distribution. The members of the Calliphoridae are also the most common cause of Korea-acquired myiasis. Among 6 reported cases of myiasis, 5 were caused by
Lucilia species with the remaining one by the result of a
Phormia species infection [
8-
13].
Myiasis caused by
L. sericata can be categorized as accidental myiasis in the ecological classification [
14]. Free-living
Lucilia larvae can cause a pathological reaction when coming in incidental contact with the host. In an analysis by Sherman [
15] of maggots from 20 medical centers in the US, the most common species isolated was
L. sericata, which was isolated from 71% (30/42) of the patients [
15]. However, when Sherman [
15] compared his results to 72 previous reports that described 137 cases of US-acquired human myiasis from1960 to 1995, the results greatly differed. The most common causative species was the rodent botfly (
Cuterebra species), followed by the screwworm fly (
Cochliomyia hominivorax), and sheep botfly (
Oestrus ovis).
L. sericata was identified only in 8 cases (5.8%). This discrepancy was assumed to be a result of underreporting of non-invasive myiasis. One reason for this was that non-invasive myiasis was considered unworthy of reporting [
15]. Lukin's prospective study [
16] in Australia also showed similar results;
Lucilia cuprina was the causative agent in 12 out of 14 recorded cases from 1986 to 1988 in Brisbane.
L. cuprina is well known as the predominant species in subtropical regions, while
L. seretica is dominant in cool temperate areas. The fact that 5 cases of myiasis acquired in Korea were caused by
Lucilia species is in line with these reports.
We suspected that the present case was a nosocomial infection. The patient had spent 8 days in the ER, and the maggots were found on the day of admission to the ICU. Based on
L. sericata's life cycle, the infection was most likely acquired in the ER [
17]. Lukin [
16] and Sherman [
15] reported 5% and 43% of nosocomial infections in North America and Australia, re-spectively. A review of myiasis acquired in Korea, however, revealed that 4 of 6 cases were nosocomial, or highly suspected to be nosocomial. Flies usually attack patients who are immo-bile, injured, or severely ill, as was the case with the present patient during the summer. Therefore, special care must be taken to secure the hygiene of environments such as the ER and ICU for patients with predisposing factors for myiasis.
In conclusion, although L. sericata is not an obligatory parasite, it has a high probability of causing accidental myiasis. Since myiasis can exacerbate the severity of a disease or be fatal to patients, patients with predisposing factors such as advanced age, debilitating conditions, peripheral vascular diseases, chronic wounds, paralysis or immobility, and mental debility should be carefully protected from flies to prevent accidental and nosocomial myiasis.
References
- 1. Sharma A. Oral myiasis is a potential risk in patients with special health care needs. J Glob Infect Dis 2012;4:60-61.
- 2. Chan JC, Lee JS, Dai DL, Woo J. Unusual cases of human myiasis due to Old World screwworm fly acquired indoors in Hong Kong. Trans R Soc Trop Med Hyg 2005;99:914-918.
- 3. Kumar GV, Sowmya G, Shivananda S. Chrysomya bezziana oral myiasis. J Glob Infect Dis 2011;3:393-395.
- 4. Stevens JR, Wallman JF. The evolution of myiasis in humans and other animals in the Old and New Worlds (part I): phylogenetic analyses. Trends Parasitol 2006;22:129-136.
- 5. Derraik JG, Heath AC, Rademaker M. Human myiasis in New Zealand: imported and indigenously-acquired cases: the species of concern and clinical aspects. N Z Med J 2010;123:21-38.
- 6. Szpila K. In Amendt J, Goff MI, Campobasso CP, Grassberger M eds, Key for the identification of third instars of European blowflies (Diptera: Calliphoridae) of forensic importance. Current concepts in forensic entomology. 2010, New York, USA. Springer; pp 43-56.
- 7. Velásquez Y, Magaña C, Martínez-Sánchez A, Rojo S. Diptera of forensic importance in the Iberian Peninsula: larval identification key. Med Vet Entomol 2010;24:293-308.
- 8. Chung PR, Jung Y, Kim KS, Cho SK, Jeong S, Ree HI. A human case of internal myiasis in Korea. Korean J Parasitol 1996;34:151-154. (in Korean).
- 9. Cho JH, Kim HB, Cho CS, Huh S, Ree HI. An aural myiasis case in a 54-year-old male farmer in Korea. Korean J Parasitol 1999;37:51-53.
- 10. Joo CY, Kim JB. Nosocomial submandibular infections with dipterous fly larvae. Korean J Parasitol 2001;39:255-260.
- 11. Choi EG, Lim DM, Na MJ, Yang JM, Lee YH, Lee WY. A case of internal myiasis of the respiratory system associated with pneumonia. Tuberc Respir Dis 2002;53:650-655. (in Korean).
- 12. Kim JS, Seo PW, Kim JW, Go JH, Jang SC, Lee HJ, Seo M. A nasal myiasis in a 76-year-old female in Korea. Korean J Parasitol 2009;47:405-407.
- 13. Kim JS, Kim JW, Lee HJ, Lee IY, Oh SA, Seo M. Ophthalmomyiasis caused by a Phormia sp. (Diptera: Calliphoridae) larva in an enucleated patient. Korean J Parasitol 2011;49:173-175.
- 14. Francesconi F, Lupi O. Myiasis. Clin Microbiol Rev 2012;25:79-105.
- 15. Sherman RA. Wound myiasis in urban and suburban United States. Arch Intern Med 2000;160:2004-2014.
- 16. Lukin LG. Human cutaneous myiasis in Brisbane: a prospective study. Med J Aust 1989;150:237-240.
- 17. Clark K, Evans L, Wall R. Growth rates of the blowfly, Lucilia sericata, on different body tissues. Forensic Sci Int 2006;156:145-149.
Fig. 1Dorsal view of third instars larvae of Lucilia sericata isolated from the patient's oral cavity. The larvae showed the typical maggot-like body shape.

Fig. 2Light microscopic and SEM views of the surface of a maggot. The cephalic segment had 1 pair of antennae with 2 sensory papillae for each, and the buccal cavity had 1 pair of hooks (A). The anus had 1 pair of anal papillae (B). The body was covered with many tiny, short spines (ventral and dorsal side, C and D). The spines were arranged in distinct bands around the body showing clearly defined spine bands (C, D). The spines were sharp with a single point (E, F). a; antenna, p; papilla, h; hook.
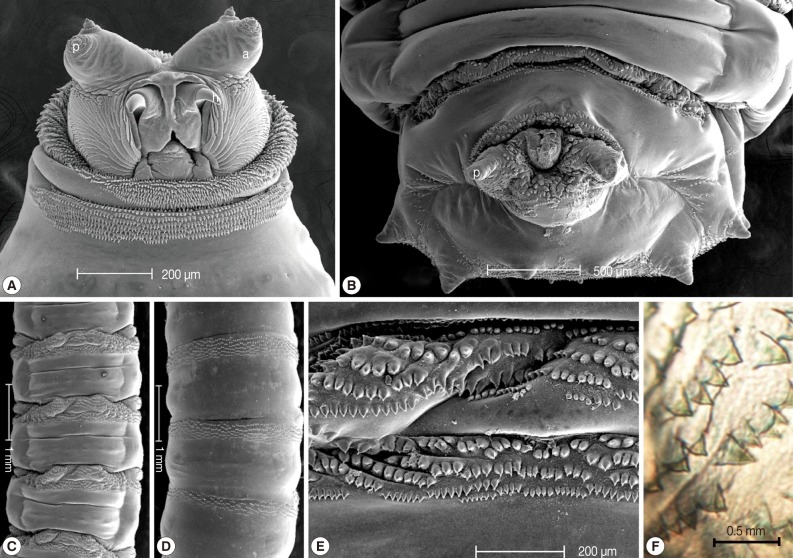
Fig. 3A light microscope image of the anterior spiracle showing a fan-like structure with 8 lobes (L).

Fig. 4Light microscopic and SEM views of the posterior part of the maggot. (A) The posterior spiracles (ps) were not positioned within a cavity. (B) The shape of the posterior spiracles was approximately round surrounded by a complete peritremal ring (pm) with no gap. (C) The button (b) presented on both spiracles and the 3 slits (s) in each posterior spiracle were straight. (D) The distances (d1, d2, and d3) of the dorsal papillae (p1), median papillae (p2), and outer papillae (p3) in the 12th segment were similar (D).
Citations
Citations to this article as recorded by

- Temperature-dependent development of Musca domestica (Diptera: Muscidae) and posterior spiracle–based substaging of third instar larvae
Ziyan Liu, Tae Mo Kang, Jieun Park, Kwang Soo Ko, Young Kyu Park, Seong Hwan Park
Forensic Science International.2026; 380: 112801. CrossRef - Imported parasitic diseases in the Republic of Korea: status and issues
Jong-Yil Chai
Journal of the Korean Medical Association.2025; 68(1): 52. CrossRef - Oral and Nasal Myiasis in Two Patients Hospitalized in the Intensive Care Unit: Diagnosis and Clinical Significance of Cases
Nilgün Savaş, Mehmet Aykur
Indian Journal of Otolaryngology and Head & Neck Surgery.2024; 76(5): 4677. CrossRef - The Price of Hospital Reshaping: Nasal Myiasis Caused by Flesh Fly (Diptera: Sarcophagidae) in Reallocated COVID-19 Intensive Care Unit
Vladimir Dolinaj, Jasmina Grujić, Davor Križanović, Aleksandar Potkonjak, Thomas Pape, Pavle Banović
Healthcare.2023; 11(11): 1533. CrossRef - Oral myiasis: Analysis of cases reported in the English literature from 1990 to 2020
Juliana Bianchi Souza dos Passos, Luiza Vale Coelho, José Alcides Almeida de Arruda, Leni Verônica de Oliveira Silva, Isabella Bittencourt do Valle, Mariana de Souza Santos, Eugênia Leal de Figueiredo, Lucas Guimarães Abreu, Ricardo Alves Mesquita
Special Care in Dentistry.2021; 41(1): 20. CrossRef - Aspiration as a Novel Technique to Address Facial and Periocular Myiasis
Jonathan E. Lu, Sukriti Mohan, Margaret L. Pfeiffer, Bhavishya S. Clark, Jessica R. Chang
Ophthalmic Plastic & Reconstructive Surgery.2021; 37(5): e172. CrossRef - Orbital myiasis
Yan-Ling Huang, Lu Liu, Hao Liang, Jian He, Jun Chen, Qiao-Wen Liang, Zhi-Yuan Jiang, Jian-Feng He, Min-Li Huang, Yi Du
Medicine.2020; 99(4): e18879. CrossRef - Wettability Property In Natural Systems: A Case of Flying Insects
J. Sackey, B. T. Sone, K. A. Dompreh, M. Maaza
MRS Advances.2018; 3(42-43): 2697. CrossRef - Oral Myiasis Affecting Gingiva in a Child Patient: An Uncommon Case Report
Fareedi Mukram Ali, Kishor Patil, Sanjay Kar, Atulkumar A. Patil, Shabeer Ahamed
Case Reports in Dentistry.2016; 2016: 1. CrossRef - Maggot debridement therapy as primary tool to treat chronic wound of animals
Vijayata Choudhary, Mukesh Choudhary, Sunanda Pandey, Vandip D. Chauhan, J. J. Hasnani
Veterinary World.2016; 9(4): 403. CrossRef - Human identification by lice: A Next Generation Sequencing challenge
Elena Pilli, Alessandro Agostino, Debora Vergani, Elena Salata, Ignazio Ciuna, Andrea Berti, David Caramelli, Simonetta Lambiase
Forensic Science International.2016; 266: e71. CrossRef - Canine Wound Myiasis Caused by Lucilia sericata (Diptera: Calliphoridae) in Korea
Seongjun Choe, Dongmin Lee, Hansol Park, Hyeong-Kyu Jeon, Hakhyun Kim, Ji-Houn Kang, Cha-Ho Jee, Keeseon S. Eom
The Korean Journal of Parasitology.2016; 54(5): 667. CrossRef - A Case of Aural Myiasis in Chronic Alcoholism Patient
Sung Hoon Jung, Jae Hwan Jung, Seok Hyun Kim, Yang Jae Kim
Journal of Clinical Otolaryngology Head and Neck Surgery.2016; 27(2): 332. CrossRef - Scanning Electron Microscopy Investigations of Third-Instar Larva of Cordylobia rodhaini (Diptera: Calliphoridae), an Agent of Furuncular Myiasis
M. Pezzi, R. Cultrera, M. Chicca, M. Leis
Journal of Medical Entomology.2015; 52(3): 368. CrossRef - A Case of Endoscopic Removal of Nasal Myiasis in Cerebral Infarction Patient
Jung-Uk Han, Sang Hyok Suk, Jun Sick Im, Bo Young Kim
Journal of Rhinology.2015; 22(1): 51. CrossRef - Mass Rearing and Life Table Attributes of Two Cyclorrhaphan Flies, Lucilia
sericata Meigen (Diptera: Calliphoridae) and Musca domestica L. (Diptera:
Muscidae) under Laboratory Conditions
Vahid Saleh, Aboozar Soltani, Tahere Dabaghmane, Hamzeh Alipour, Kourosh Azizi, Mohammad Djaefar Moemenbell
Journal of Entomology.2014; 11(5): 291. CrossRef - Flies and the mouth
Yazan Hassona, Crispian Scully, Miranda Aguida, Oslei Paes de Almeida
Journal of Investigative and Clinical Dentistry.2014; 5(2): 98. CrossRef - A Case of Nosocomial Nasal Myiasis in Comatose Patient
Sung Jae Heo, Mi Jin Lee, Chang Mook Park, Jung Soo Kim
Korean Journal of Otorhinolaryngology-Head and Neck Surgery.2013; 56(10): 664. CrossRef