Abstract
In a previous study, the author developed a method for separation of the tegument of spargana (plerocercoids of Spirometra mansoni) from the parenchyme using urea. The present study, as a next step, was performed to evaluate which molecules are present in the outer tegument. Two major proteins, 180 and 200 kDa, are present in the tegument and we could make polyclonal antibodies against these molecules. Their immunolocalization was processed and the outermost layer of the spargana showed strong positive staining. Conclusively, we could confirm that the 180 and 200 kDa molecules might be tightly bound membrane proteins in the tegument of spargana.
-
Key words: sparganum, syncytial layer, polyclonal antibody, immunohistochemistry
The sparganum (plerocercoid of
Spirometra mansoni) is a tissue-invading parasite in the intermediate hosts, including humans, causing subcutaneous masses or infrequently to other organs, such as the central nervous system [
1,
2]. Therefore, the outer tegumental layers of spargana should have crucial roles in invasion and host antibody production. Studies on the parasitic tegument should provide several clues of host-parasite interactions, pharmacologically drug targets, and advanced diagnostic molecules [
3]. Also, the tegument of parasites may be involved as an initial signal for antigenicity to the host. Therefore, studies on the potent antigenic molecules for antibody production should be processed by the tegumental layer of spargana [
4-
6]. In this regard, the present author has developed a method of separating the syncytial layer from the parenchyma of spargana in a previous study [
7]. The present study was performed to evaluate the protein composition of the outer tegument of spargana, and the immunolocalization probed with polyclonal antibodies against the tegument was processed.
Spargana were collected from subcutaneous tissues of snakes caught nearby Jinju in Korea. After washing with sterile saline, the worms were stored at 4℃ until infection into mice. A total of 5 spargana were infected orally to each of 5 BALB/c mice. After 4 weeks, infected mice were sacrificed and worms were collected from the subcutaneous tissues of the mice.
The outer tegument was separated from the sparganum by the method of Yang [
7]. After collection, the tegument was washed with PBS 3 times. Protein extracts of the tegument were prepared by 2 different ways; one is homogenization in 10 times volume of PBS and the other is using protein extraction solution (PRO-PREP; Intron, Seoul, Korea) containing the ionic detergent of SDS. All extracts produced were preserved in 4℃ for next experiments.
Equal volumes of both PBS and detergent tegumental extracts were mixed with Freund's incomplete adjuvant (Sigma, St. Louis, Missouri, USA) and injected intraperitoneally into BALB/c mice. Three weeks later, this was repeated again with Freund's complete adjuvant (Sigma). Ether anesthetized mice were bled from the tail vein, and serum was stored in refrigerator.
The tegumental protein extracts were separated by 7.5-15% SDS-PAGE and then transferred onto polyvinylidene difluoride (PVDF; Millipore, Bedford, Massachusetts, USA) membranes. Membrane strips were then reacted with polyclonal antibody at a 1 : 100 dilution in casein buffer (0.5% casein, 20 mM Tris, 150 mM NaCl). The membranes were then reacted with peroxidase conjugated anti-mouse IgG antibody (Fc specific; Cappel, Westchester, Pennsylvania, USA) diluted at 1 : 2,000. Finally, colors were developed with 4-chloro-1-naphthol (Sigma).
After separation of the syncytial layer, spargana were fixed in 10% neutral buffered formalin and embedded in paraffin. Each paraffin block was used for 2 spargana and staining was processed triplicate for its reproducibility. For immunohistochemistry, the avidin-biotin complex (ABC) staining system (Santa Cruz, California, USA) was used. Briefly, the polyclonal mice sera against the tegumental extract, diluted at 1 : 100, were incubated with worm sections for 3 hr at room temperature. After then, the stain was followed according to the manufacturer's protocol. Finally, the reaction was developed with a substrate, diaminobenzidine (DAB) and counterstained using hematoxylin.
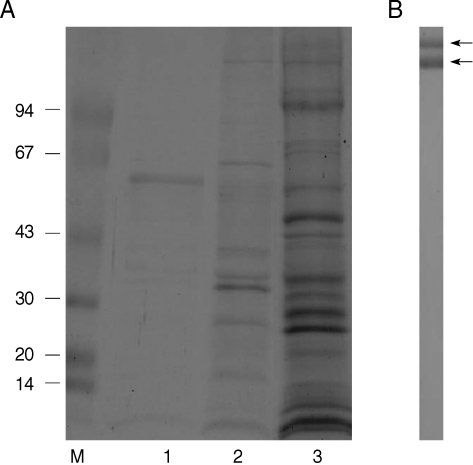
As shown in
Fig. 1A, PBS extracts of the tegument were represented into a 60 kDa major single band and faint proteins, including 10, 33, and 36 kDa molecules in SDS-PAGE (lane 1). However, numerous molecules were noticed in the detergent contained protein extraction (lane 2). About 16 protein bands were noticed ranging from 10 to 200 kDa molecules.
Polyclonal antibodies against detergent extracted proteins of the tegument was confirmed by immunoblot (
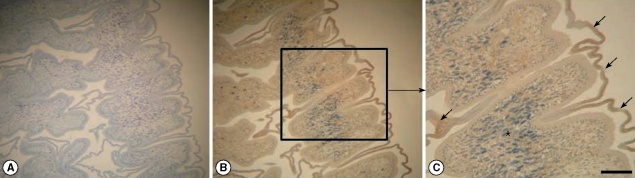
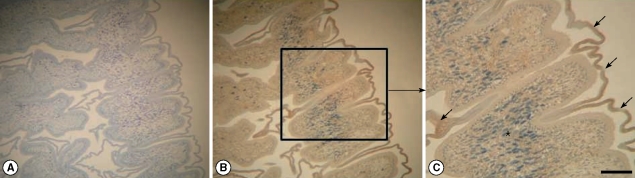
Fig. 1B) and 2 major bands of 180 and 200 kDa molecules were reacted with transferred proteins. Polyclonal antibodies reacting with other proteins were not generated by immunization on mice. However, immunoblots using polyclonal antibodies against PBS extracted proteins did not detect any specific tegumental proteins (data not shown). Using polyclonal antibodies, its immunolocalization was processed by immunohistochemistry. In
Fig. 2B and C, strong positive reactions are developed in the outer tegument of the sparganum.
Analysis of tegumental proteins has been evaluated by several detergents, such as digitonin [
8,
9], saponin [
10], or Triton-X [
11]. Kim and Cho [
9] reported that the tegumental fraction of
Paragonimus westermani collected by 0.1% digitonin was difficult to homogenize by the Potter-Elvehjem glass teflon homogenizer, but easily solubilized in the detergent contained lysis buffer by sonication. In this study, the same phenomenon was noticed as shown in
Fig. 1A. In SDS-PAGE, soluble proteins of the outer tegument were not easy to detect in PBS extracts by homogenization, but numerous proteins were easily detected in detergent treated extracts. This finding suggested that the major tegumental proteins, such as 180 and 200 kDa molecules, may be tightly bound membrane proteins as structural proteins.
In the tegumental studies of
Schistosoma sp, it has been known that there are unique structures and large numbers of proteins (28%) in the tegument which lack sequence similarity to any other proteins in other species [
12]. These unique proteins might be correlated with specific structures and functions of the schistosomal tegument [
12]. Therefore, the tegumental proteins having few similarities to the host proteins should be expected to be highly antigenic, because tegumental proteins are the initial triggering molecules by a contact with the host [
13]. Also, it is well known that there are relatively few schistosome tegumental proteins which have functions in nucleotide and protein synthesis. It has been confirmed that this is correlated with the absence of nuclei in the distal cytoplasm of the tegument [
12].
In immunization with detergent extracted tegumental proteins, we could collect mice sera of strong reactions with 180 and 200 kDa molecules (
Fig. 1B) but unfortunately, we could not review this phenomenon during the production of polyclonal antibodies against other molecules. Finally, we could confirm their localization at the outer tegument of spargana by immunohistochemistry (
Fig. 2B, C). In the previous immunoblot study, high molecular masses were detected in the early phase of mouse sparganosis [
14]. Therefore, 180 and 200 kDa molecules may be expected to sparganum-specific tegumental proteins which are the first contact molecules when infected to the host.
Recently, van Balkom et al. [
12] reported that a total of 740 expressed schistosomal proteins were identified, and 179 of the proteins were detected in both the tegument and the worm body. Of them, 43 proteins were specifically located in the tegument and these tegument specific proteins were obviously a fraction of the total tegumental proteins. In this study, it was confirmed that 2 specific molecules of 180 and 200 kDa are specifically detected in the tegument of spargana. Further studies on the detergent solubilized other molecules of the tegument in spargana should be processed whether their functions may be both structural and antigenic.
ACKNOWLEDGEMENTS
The author thanks Mr. Je-Young Ryu for his technical assistances. This research was supported by the grants for Sabbatical from the Ewha Womans University, 2009.
References
- 1. Chang KH, Chi JG, Cho SY, Han MH, Han DH, Han MC. Cerebral sparganosis: analysis of 34 cases with emphasis on CT features. Neuroradiology 1992;34:1-8.
- 2. Cho SY, Bae JH, Seo BS, Lee SH. Some aspects of human sparganosis in Korea. Korean J Parasitol 1975;13:60-77.
- 3. Wilson RA, Curwen RS, Braschi S, Hall SL, Coulson PS, Ashton PD. From genomes to vaccines via the proteome. Mem Inst Oswaldo Cruz 2004;99:45-50.
- 4. Kim LS, Kong Y, Kang SY, Cho SY. Immunohistochemical localization of 36 and 29 kDa proteins in sparganum. Korean J Parasitol 1992;30:25-31.
- 5. Ohnishi Y, Takakura Y, Yoshimura H, Tsubota N. Antibody production in experimental sparganosis of rabbits: agglutination and indirect fluorescent antibody techniques. Jpn J Parasitol 1986;35:25-33.
- 6. Kim CH, Choi WS. Immunohistochemical observation on the antigens inducing IgG antibodies and IgM against sparganum. Korean J Parasitol 1991;29:339-353.
- 7. Yang HJ. Separation of the syncytial layer of spargana using urea. Korean J Parasitol 2009;47:69-71.
- 8. Mills GL, Coley SC, Williams JF. Lipid and protein composition of the surface tegument from larvae of Taenia taeniformis. J Parasitol 1984;70:197-207.
- 9. Kim SI, Cho SY. Protein composition and antigenicity of the tegument from Paragonimus westermani. Korean J Parasitol 1993;31:269-276.
- 10. Kusel JR. Protein composition and protein synthesis in the surface membranes of Schistosoma mansoni. Parasitology 1972;65:55-69.
- 11. Oak JA, Cain GD, Mower DA, Raj RK. Disruption and removal of the tegument from Schistosoma mansoni with Triton X-100. J Parasitol 1981;67:761-775.
- 12. van Balkom BW, van Gestel RA, Brouwers JFHM, Krijgsveld J, Tielens AGM, Heck AJ, van Hellemond JJ. Mass spectrometric analysis of the Schistosoma mansoni tegumental sub-proteome. J Proteome Res 2005;4:958-966.
- 13. Jones MK, Gobert GN, Zhang L, Sunderland P, McManus DP. The cytoskeleton and motor proteins of human schistosomes and their roles in surface maintenance and host-parasite interactions. Bioessays 2004;26:752-765.
- 14. Chung YB, Kong Y, Yang HJ, Cho SY. IgG antibody responses in early experimental sparganosis and IgG subclass responses in human sparganosis. Korean J Parasitol 2000;38:145-150.
Fig. 17.5-15% SDS-PAGE of tegumental proteins of spargana and production of polyclonal antibodies. (A) Note the 60 kDa major single band in PBS extracts of the tegument (lane 1) and numerous molecules by detergent extracted proteins (lane 2) compared with soluble crude extracts (lane 3) of the sparganum. (B) Polyclonal antibodies against detergent extracted proteins were reacted with 2 major bands of 180 and 200 kDa molecules.
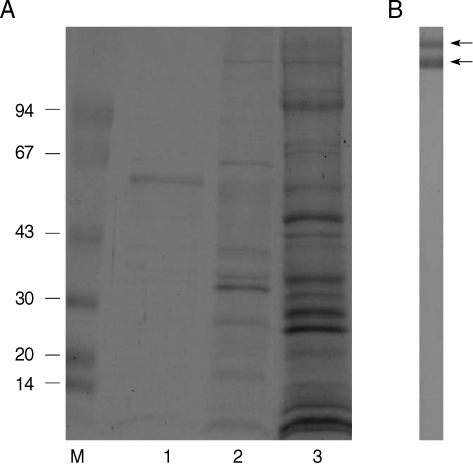
Fig. 2Immunohistochemistry by polyclonal antibodies against tegumental molecules in spargana. Strong positive reactions are observed in the outer tegument of a sparganum (arrow). No other organelles, including calcareous corpuscles (*), show a positive reaction. (A) negative control. × 100 magnification; (B) and (C): stain with polyclonal antibodies. × 100 and × 200 magnifications, respectively (Scale bar=100 µm).
Citations
Citations to this article as recorded by

- Identification of an enolase gene and its physiological role in Spirometra mansoni
Pei Liang, Xiuji Cui, Ruijia Fu, Peng Liang, Gang Lu, Dayong Wang
Parasitology Research.2021; 120(6): 2095. CrossRef