Abstract
The present study was performed to observe histopathological changes in tissues of Bithynia siamensis goniomphalos (Gastropoda, Bithyniidae) incubated in crude extract solutions of camellia (Camellia oleifera) seed and mangosteen (Garcinia mangostana) pericarp, and furthermore to estimate the molluscicidal effects of 2 plant substances. Substantial numbers of bithyniid snails were incubated in various concentrations of 2 plant solution for 24 hr. As the positive control, snails incubated in various concentrations of niclosamide, a chemical molluscicide, were used. The histopathological findings were observed in sectioned snail specimens of each experimental and control groups. The results showed that both camellia and mangosteen extracts had molluscicidal effects at 24 hr with 50% lethal concentration (LC50) at concentrations of 0.003 and 0.002 g/ml, respectively, while niclosamide had LC50 at concentrations 0.599 ppm. B. siamensis goniomphalos snail tissues (foot, gill, and digestive system) showed disruption of columnar muscle fibers of the foot, reduction of the length and number of gill cilia, numerous mucous vacuoles, and irregularly shaped of epithelial cells. Irregular apical and calciferous cells, dilatation of the digestive gland tubule, and large hemolymphatic spaces, and irregular apical surfaces, detachment of cilia, and enlargement of lysosomal vacuoles of epidermis were also shown in all groups. By the present study, it is confirmed that 2 plants, camellia and mangosteen, are keeping some substance having molluscicidal effects, and histopathological findings obtained in this study will provide some clues in further studies on their action mechanisms to use them as natural molluscicides.
-
Key words: Opisthorchis viverrini, Bithynia siamensis goniomphalos, molluscicidal effect, Camellia oleifera, Garcinia mangostana, niclosamide, Thailand
INTRODUCTION
It is well known that
Bithynia siamensis goniomphalos (Gastropoda, Bithyniidae) snails are an important first intermediate host of many trematodes, including the human liver fluke,
Opisthorchis viverrini [
1,
2]. General methods for preventions and controls of human parasitic diseases are health education, diagnostic technique, chemotherapy, environmental management, sanitation, and water supply, including the snail control [
3]. However, each method has limitations such as misunderstanding, economics, geographical characteristics, drug resistance, and so on. Another choice of parasite control is to cut the life cycle which is focused in snails.
They are many methods to control snails, physical control [
4], ecological control [
5], and biological control [
6]. These previous methods could not be used now or had less effectiveness; therefore, chemical control methods remain for use in the community. Although many molluscicides had been reported such as copper sulfate [
7], endosulfan [
8], and bayluscide [
9] but they had toxic effects on fish, water plants, and small organisms. Many researchers tried to use others including plants which are available in the community and less toxic effects on humans, aquatic animals, and plants.
It has been known that some plants, such as the soap berry endod (
Phytolacca dodecandra), the Indian rosewood (
Dalbergia sissoo), the headed savory (
Thymus capitatus), and the white horehound (
Marrubium vulgare), retain the molluscicidal substance and then they were traditionally used in the snail control of schistosomiasis [
10-
12]. Camellia (
Camellia oleifera) seed is one of the plants that department of fisheries recommended to use for killing snails. In tropical countries including Thailand, mangosteen (
Garcinia mangostana) can be found in the raining season and its pericarp becomes a waste product. Several pharmacologists or researchers want to clarify its properties. They found that
G. mangostana pericarp had many properties such as antibacterial [
13], antifungal [
14], and anthelmintic activities [
15]. In the present study, histopathological changes were observed in tissues of
B. siamensis goniomphalos incubated in crude extract solutions of camellia seed and mangosteen pericarp, and to estimate the molluscicidal effects of the 2 plant substances.
MATERIALS AND METHODS
Snails used, B. siamensis goniomphalos
B. siamensis goniomphalos snails were collected from a freshwater reservoir in an endemic area of Khon Kaen, northeast Thailand.
B. siamensis goniomphalos snails were identified using standard morphological guidelines [
16,
17]. Snails were allowed to acclimatize under laboratory conditions for 3 days before experiments. Only active snails were used in the experiment.
Preparation of mangosteen pericarp solution
Samples of fresh mangosteen pericarp were washed and incubated at 60℃ for 24 hr. Then, 50 g of dried mangosteen peel was mixed well with 500 ml distilled water using a magnetic stirrer, and filtered through a 0.45 µm filter paper.
Preparation of camellia seed solution
Camellia seed (10% saponin) was purchased from Ekyongyut Co. (Samut Sakhon, Thailand). Ten g of camellia seeds were dissolved in 100 ml of distilled water and then mixed well using a magnetic stirrer. This stock solution was prepared for 1:10 v/v, 1:100 v/v, 1:1,000 v/v, 1:10,000 v/v, 1:100,000 v/v, and 1:1,000,000 v/v, respectively.
Preparation of niclosamide solution
Niclosamide was purchased from Sigma-Aldrich (Beijing, China), and 0.001 mg of niclosamide was dissolved in 100 ml distilled water and mixed well using a magnetic stirrer. This was used for the stock solution (1:1,000,000), and prepared at 0.0156, 0.0313, 0.0625, 0.125, 0.250, 0.375, 0.500, 0.625, 0.750, 0.875, and 1 ppm.
Criteria for dead snails and data analysis
The criteria used for dead snail identification followed previous reports [
18-
21]. These included the snails showing no reaction after probing with a needle to elicit typical withdrawal movements, loss of their opercula and lack of movement, sinking to the bottom of the container and complete retraction into their shells, discoloration, and immobility, i.e. gentle prodding of the cephalopedal mass with a blunt plastic probe elicited no muscular movements or contraction.
To compare the efficacy of camellia, mangosteen extracts, and niclosamide, B. siamensis goniomphalos were incubated with various concentrations of camellia, mangosteen, and niclosamide solution. The concentrations of crude extracts of mangosteen and camellia were 0.1, 0.01, 0.001, 0.0001, 0.00001, and 0.000001 g/ml. The concentrations of niclosamide solution were 0.0156, 0.0313, 0.0625, 0.125, 0.250, 0.375, 0.500, 0.625, 0.750, 0.875, and 1.0 ppm. Each concentration was examined in triplicates. Lethal concentration 50% (LC50) and 90% (LC90) values of each group at 24 hr was calculated using the Probit analysis (SPSS 16.0).
Histopathological observations
After 24 hr exposure, only live
B. siamensis goniomphalos snails from each group (untreated control, camellia-treated, mangosteen-treated, and niclosamide-treated), were processed for analysis of histopathological changes by hematoxylin and eosin staining, following previous reports [
22-
24]. In brief,
B. siamensis goniomphalos snails were fixed with buffered 10% formalin solution for 1 week and then washed with PBS solution for 15 min 3 times. The shell was removed and all snails were dehydrated in a graded ethanol series and cleared with xylene solution for 2 hr; the tissues were then infiltrated with paraffin. Paraffin-embedded snail samples in each experimental group were sliced in 4 µl thickness with a microtome (Thermoscientific, Walldorf, Germany). Each sectioned specimen was placed on a slide glass and stained with hematoxylin and eosin after deparaffinization.
The stained samples were observed using a light microscope and photographed using the NIS-Elements 3.2 imaging software (Nikon, Tokyo, Japan).
RESULTS
Molluscicidal effects in LC50 and LC90
The concentrations of both extracts that could kill 50% and 90% of B. siamensis goniomphalos snails after 24 hr were quite similar by 0.002 and 0.212 g/ml for mangosteen and 0.003 and 0.363 g/ml for camellia. For niclosamide, the LC50 and LC90 were very low (0.599 and 2.356 ppm, respectively).
Macroscopic findings
The macroscopic visible reactions of the snails exposed to crude extracts of camellia, mangosteen, and niclosamide were as follows: After exposure to camellia extracts at concentrations of 0.0001, 0.001, 0.01, and 0.1 g/ml, snails moved back within their shells, closed their operculum, and did not move within 1 hr (at concentrations of 0.01 and 0.1 g/ml). Most snails could attach the container for 12 hr. After exposure to mangosteen extracts at concentrations of 0.0001, 0.001, 0.01, and 0.1 g/ml, snails moved back within their shells, closed their operculum, and could not move at different time points (12 hr, 6 hr, 1 hr, and 10 min, respectively) at each concentration. Clear mucous was observed in both groups of snails in concentrations of 0.01 and 0.1 g/ml. At 0.0001 and 0.001 g/ml, snails could open their operculum again after 5-10 min and move again. After exposure to niclosamide solution at concentrations of 0.500, 0.625, 0.750, 0.875, and 1.0 ppm, snails moved back within their shells, closed their operculum, and did not move at 1 hr, 30 min, 20 min, 10 min, and 5 min, respectively.
Pathological changes after treatment with camellia, mangosteen, and niclosamide
Controls
No pathological changes were observed in the digestive gland and foot of the control snails.
Foot
Based on observation of the normal morphology of muscle cell types (
Fig. 1A, B) in the untreated control group, the foot tissues of snails in camellia, mangosteen, and niclosamide-treated groups consisted of protein cells, pigment cells, columnar muscle fibers, and epithelium (
Fig. 1C-H). These cells in the foot were the targets of toxic activity of camellia, mangosteen, and niclosamide. Interestingly, exposure to camellia, mangosteen, and niclosamide, columnar muscle fibers in the foot were disrupted. In addition, protein and pigment cells were found to be concentrated.
Gill
In the gill of untreated snails, cells of gill filaments were columnar in shape and bore long cilia. Some mucus-secreting goblet cells could also be found in gill filaments (
Fig. 2A, B). The snail gills in camellia and mangosteen-treated groups appeared to be more severe than the niclosamide-treated group. After camellia, mangosteen, and niclosamide exposure, the gill epithelium showed a reduction in the length and number of cilia, the mucous vacuoles appeared to be more numerous, epithelial cells were irregularly shaped, and hypertrophy of nuclei was observed. In addition, the structure of the epithelial layer was severely destroyed, with condensed nuclei and irregularly shaped cells visible (
Fig. 2C-H).
Digestive gland tubule
The digestive gland tubule of control snails consisted of regular apical digestive cells, basophil cells, and calciferous cells (
Fig. 3A, B). Snails treated with either camellia or mangosteen and niclosamide showed a small number of irregular apical and calciferous cells, dilatation of the digestive gland tubule, and large hemolymphatic spaces (
Fig. 3C-H). Moreover, the digestive gland tubule in niclosamide-treated snails showed more severe pathology by loss of digestive gland tubule texture (
Fig. 3G, H).
Epithelial lining of the digestive tract
The epithelial lining of the digestive tract of control snails was characterized by regular apical surfaces, by observation of columnar cells with typical cilia and nucleus morphology (
Fig. 4A, B). Both camellia and mangosteen-treated groups showed an increase of vacuolation in digestive columnar cells, and loosening of cilia and nucleus morphology (
Fig. 4C-F). Although, epithelial lining of the digestive tract in the group of niclosamide exposure was regular apical surfaces, by observation of columnar cells with typical cilia and nucleus morphology but remained the epithelial lining texture (
Fig. 4G, H).
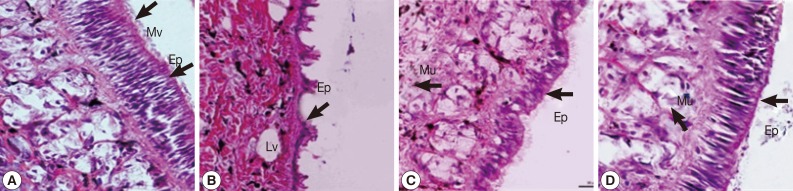
Epidermis
The epidermal cells of control snails were characterized by regular apical surfaces (
Fig. 5A). After camellia and mangosteen exposure, epithelial cells mainly showed irregular apical surfaces, detachment of cilia, and enlargement of lysosomal vacuoles (
Fig. 5B, C). The epidermal cells of niclosamide-exposure group (
Fig. 5D) were similar with those of control snails.
DISCUSSION
The present study showed that camellia, mangosteen extracts, and niclosamide affected cells in the foot by relaxing muscle fibers and creating gaps between epithelial cells and connective tissue, resulting in the derangement of the cilia. The digestive tract of control
B. siamensis goniomphalos snails was lined by a ciliated columnar epithelium, consisting of columnar cells (
Fig. 4A, B) and mucus-secreting goblet cells (
Fig. 2A, B). The ciliated columnar cells were long and slender in shape, with an oval nucleus with dense heterochromatin in the basal cytoplasm. In the camellia and mangosteen-exposed snails, an enlargement of nuclei, increase of mucus, damage of cell structure and enlarged interlamellar space and loss of texture were observed (
Fig. 2C, F). Our present results were similar to the previous report [
25], which showed that snails exposed to heavy metals showed an increase in mucous cells and mucus vacuoles; this implies that mucus may contribute to the capacity for detoxification, and that the digestive tract needs more mucus to dilute the toxin from camellia and mangosteen [
26]. This result is supported by el Sawy et al. [
27] who found that these chemicals may produce an indirect reaction in the snail by causing generation of excessive mucus, reducing the permeability of the external membrane, or reacting directly with the external membrane. The loss of cilia may be caused by interaction of camellia and mangosteen with the cell membrane, inducing changes in composition, fluidity, and stability of the membrane; these are similar to the results of interactions with heavy metals reported in previous reports [
28,
29].
The digestive gland consisted of numerous blind-ending tubules that were composed of calciferous cells and basophil cells. The most common digestive cells were columnar type, with numerous vacuoles containing faintly stained inclusions. The basophil cells occurred singly or in groups and contained basophilic granules of various sizes (
Fig. 3A, B). They were secretory in nature, while the digestive cells were involved in absorption and intracellular digestion [
30]. After camellia and mangosteen exposure, the digestive gland showed a decrease of calciferous cells and dilation of digestive cells (
Fig. 3C-F). Similar calciferous cells were observed in
B. siamensis goniomphalos snails treated with the molluscicide, Bayluscide or niclosamide [
31]. The gill of control snails consisted of a longitudinal central axis from which numerous slender, pointed gill filaments extended along one side. Gill filaments were ciliated columnar epithelial cells containing a dense oval nucleus and acidophilic granules (
Fig. 2A, B). Numerous hemocytes were found in the hemolymph space (
Fig. 2A, B). After camellia seed and mangosteen exposure, the gill exhibited a loss of cilia and wider hemolymph spaces. Columnar cells showed moderate to severe alterations, such as swelling, degeneration, and loss of cells, creating large intercellular spaces (
Fig. 2C-F). The gill is a vital organ in aquatic organisms because it plays an essential role in transport of respiratory gases, and regulates osmotic and ionic balances [
32].
The observed histopathological alterations of the gill are similar to those produced by heavy metals, causing respiratory problems as well as disturbance of normal osmoregulatory and hemodynamic functions [
33]. This was similar to the previous finding [
34] which showed the effect of heavy metals on the golden apple snail (
Pomacea canaliculata). The digestive tract of the treated snails showed an increase of mucus cells and loss of cilia; most cells were dilated, and the tubule lumen became smaller. Gills of treated snails showed loss of cilia and numerous intercellular spaces among columnar cells. This was similar to the effect of the pesticide Thiodan® on
Lymnaea stagnalis snails, which caused expansion of the hemolymphatic spaces between the tubules, accumulation of amoebocytes in the hemolymphatic spaces between the tubules, and exudates in the lumen of tubules. Necrosis and increasing vacuolation within the digestive cells and atrophy in the connective tissue were apparent, and columnar muscle fibers in the foot were disrupted. In addition, the protein and pigment cells were found to be concentrated. Both camellia and mangosteen-treated groups showed detachment of ciliated cells, with enlarged lysosomal vacuoles and an irregular surface and changes also occurred in the epithelial layers [
35], which were similar to the present results (
Fig. 4C-F).
For the molluscicidal mechanisms and active compounds in each molluscide need to be clarified. However, from our study we found that camellia contained 10% saponion, and mangosteen contained α-mangostin which may affect snail tissues by interference of the respiratory tract similar to niclosamide. Therefore, after exposure to those compounds snail could not breath which affected to failure of body organs including gastrointestinal tract and foot tissues. WHO recommended using molluscides including niclosamide in the field by slowly releasing molluscide into flowing water by a drip-feed technique using a drum dispenser at narrow canal to ensure complete mixing of the chemical with the water. In another way, if we purpose to kill snails in the rice field, pond, or fish farm, we could spray camellia and mangosteen powders in the rice field or pond during rice cultivation and pond preparation. The concentrations of mangosteen in the field using may be high but mangosteen peel in Thailand is the waste product during raining season so we have a lot of this kind of waste product and could use it. However, further studies in the field are needed.
In conclusion, exposure of B. siamensis goniomphalos snails to crude extracts of either camellia or mangosteen caused destructive effects in the body tissues which was similar to niclosamide, as evidenced by the LC50 and LC90 and histopathological changes. Therefore, crude extracts of camellia and mangosteen are effective molluscides that may be used in the field or on fish farms during preparing the pond to control B. siamensis goniomphalos snails. Further studies are needed to clarify the exact substance or mechanism for snail killing. Moreover, field studies are needed to confirm the possibility of using these extracts.
Office of the Higher Education CommissionKhon Kaen UniversityKKU56
TRF Senior Research Scholar GrantRTA5580004
Animal Experimental UnitAS54301
Notes
-
We have no conflict of interest related with this study.
ACKNOWLEDGMENTS
This work was supported by the Higher Education Research Promotion and National Research University Project of Thailand, Office of the Higher Education Commission, through the Health Cluster (Shep-GMS) and Khon Kaen University Research funding (KKU56) and TRF Senior Research Scholar Grant no. RTA5580004, Thailand. We thank the Animal Experimental Unit, the Department of Research Affairs (AS54301), Faculty of Medicine, Khon Kaen University, and the Department of Parasitology for their support.
References
- 1. Herrmann KK, Sorensen RE. Seasonal dynamics of two mortality-related trematodes using an introduced snail. J Parasitol 2009;95:823-828.
- 2. De Liberato C, Scaramozzino P, Brozzi A, Lorenzetti R, Di Cave D, Martini E, Lucangeli C, Pozio E, Berrilli F, Bossù T. Investigation on Opisthorchis felineus occurrence and life cycle in Italy. Vet Parasitol 2011;177:67-71.
- 3. World Health Organization. The control of schistosomiasis. Report of WHO Expert Committee. Geneva, Switzerland. WHO; 1985, pp 58-59.
- 4. Hoffman GL. Control methods for snail-borne zoonoses. J Wildl Dis 1970;6:262-265.
- 5. Wang WL, Zhang HZ, Cai ZD, Qin Q, Liu FC, Xu XJ, Wei FH, Zheng J. Evaluation on schistosomiasis control effect of the intervention measures adapted to the ecological environment changes in Jiang Han Plain due to establishment of the Three Gorges dam. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi 2007;25:114-119. (in Chinese).
- 6. Hung NM, Duc NV, Stauffer JR Jr, Madsen H. Use of black carp (Mylopharyngodon piceus) in biological control of intermediate host snails of fish-borne zoonotic trematodes in nursery ponds in the Red River Delta, Vietnam. Parasit Vectors 2013;6:142.
- 7. Reddy A, Ponder EL, Fried B. Effects of copper sulfate toxicity on cercariae and metacercariae of Echinostoma caproni and Echinostoma trivolvis and on the survival of Biomphalaria glabrata snails. J Parasitol 2004;90:1332-1337.
- 8. Otludil B, Cengiz EI, Yildirim MZ, Unver O, Unlü E. The effects of endosulfan on the great ramshorn snail Planorbarius corneus (Gastropoda, Pulmonata): a histopathological study. Chemosphere 2004;56:707-716.
- 9. Dai JR, Coles GC, Wang W, Liang YS. Toxicity of a novel suspension concentrate of niclosamide against Biomphalaria glabrata. Trans R Soc Trop Med Hyg 2010;104:304-306.
- 10. Abebe F, Erko B, Gemetchu T, Gundersen SG. Control of Biomphalaria pfeifferi population and schistosomiasis transmission in Ethiopia using the soap berry endod (Phytolacca dodecandra), with special emphasis on application methods. Trans R Soc Trop Med Hyg 2005;99:787-794.
- 11. Adenusi AA, Odaibo AB. Laboratory assessment of molluscicidal activity of crude aqueous and ethanolic extracts of Dalbergia sissoo plant parts against Biomphalaria pfeifferi. Travel Med Infect Dis 2008;6:219-227.
- 12. Salama MM, Taher EE, El-Bahy MM. Molluscicidal and mosquitocidal activities of the essential oils of Thymus capitatus Hoff et Link. and Marrubium vulgare L. Rev Inst Med Trop Sao Paulo 2012;54:281-286.
- 13. Iinuma M, Tosa H, Tanaka T, Asai F, Kobayashi Y, Shimano R, Miyauchi K. Antibacterial activity of xanthones from guttiferaeous plants against methicillin-resistant Staphylococcus aureus. J Pharm Pharmacol 1996;48:861-865.
- 14. Kaomongkolgit R, Jamdee K, Chaisomboon N. Antifungal activity of alpha-mangostin against Candida albicans. J Oral Sci 2009;51:401-406.
- 15. Keiser J, Vargas M, Winter R. Anthelminthic properties of mangostin and mangostin diacetate. Parasitol Int 2012;61:369-371.
- 16. Upatham ES, Sornmani S, Kittikorn V, Lohachit C, Burch JB. Identification key for fresh and brackishwater snail of Thailand. Malacol Rev 1983;16:107-132.
- 17. TROPMED Technical Group. Snails of medical importance in Southeast Asia. Southeast Asian J Trop Med Public Health 1986;17:282-322.
- 18. Evans NA, Whitfield PJ, Squire BJ, Fellows LE, Evans SV, Millott SM. Molluscicidal activity in the seeds of Millettia thonningii (Leguminosae: Papilionoideae). Trans R Soc Trop Med Hyg 1986;80:451-453.
- 19. dos Santos AF, Ferraz PA, Pinto AV, Pinto M do C, Goulart MO, Sant'Ana AE. Molluscicidal activity of 2-hydroxy-3-alkyl-1, 4-naphthoquinones and derivatives. Int J Parasitol 2000;30:1199-1202.
- 20. Al-Zanbagi NA, Barrett J, Banaja A. Laboratory evaluation of the molluscicidal properties of some Saudi Arabian euphorbiales against Biomphalaria pfeifferi. Acta Trop 2001;78:23-29.
- 21. Teixeira T, Rosa JS, Rainha N, Baptista J, Rodrigues A. Assessment of molluscicidal activity of essential oils from five Azorean plants against Radix peregra. Chemosphere 2012;87:1-6.
- 22. Boonjaraspinyo S, Boonmars T, Aromdee C, Puapairoj A, Wu Z. Indirect effect of a turmeric diet: enhanced bile duct proliferation in Syrian hamsters with a combination of partial obstruction by Opisthorchis viverrini infection and inflammation by N-nitrosodimethylamine administration. Parasitol Res 2011;108:7-14.
- 23. Boonmars T, Wu Z, Boonjaruspinyo S, Puapairoj A, Kaewsamut B, Nagano I, Pinlaor S, Yongvanit P, Wonkchalee O, Juasook A, Sudsarn P, Srisawangwong T. Involvement of c-Ski oncoprotein in carcinogenesis of cholangiocarcinoma induced by Opisthorchis viverrini and N-nitrosodimethylamine. Pathol Oncol Res 2011;17:219-227.
- 24. Wonkchalee O, Boonmars T, Kaewkes S, Chamgramol Y, Aromdee C, Wu Z, Juasook A, Sudsarn P, Boonjaraspinyo S, Pairojkul C. Comparative studies on animal models for Opisthorchis viverrini infection: host interaction through susceptibility and pathology. Parasitol Res 2012;110:1213-1223.
- 25. Viard B, Pihan F, Promeyrat S, Pihan JC. Integrated assessment of heavy metal (Pb, Zn, Cd) highway pollution: bioaccumulation in soil, Graminaceae and land snails. Chemosphere 2004;55:1349-1359.
- 26. Triebskorn R, Telcean I, Casper H, Farkas A, Sandu C, Stan G, Colğrescu O, Dori T, Köhler HR. Monitoring pollution in River Mureş, Romania, part II: metal accumulation and histopathology in fish. Environ Monit Assess 2008;141:177-188.
- 27. el Sawy MF, Duncan J, Amer S, el Ruweini H, Brown N, Hills M. The molluscicidal properties of Ambrosia maritima L. (Compositae). A comparative field trial using dry and freshly-harvested plant material. Trop Med Parasitol 1987;38:101-105.
- 28. Riba I, Blasco J, Jiménez-Tenorio N, de Canales ML, DelValls TA. Heavy metal bioavailability and effects: II. Histopathology-bioaccumulation relationships caused by mining activities in the Gulf of Cádiz (SW, Spain). Chemosphere 2005;58:671-682.
- 29. Tanhan P, Sretarugsa P, Pokethitiyook P, Kruatrachue M, Upatham ES. Histopathological alterations in the edible snail, Babylonia areolata (spotted babylon), in acute and subchronic cadmium poisoning. Environ Toxicol 2005;20:142-149.
- 30. Abdallah AT, Moustafa MA. Accumulation of lead and cadmium in the marine prosobranch Nerita saxtilis, chemical analysis, light and electron microscopy. Environ Pollut 2002;116:185-191.
- 31. Tesana S, Thapsripair P, Thammasiri C, Prasopdee S, Suwannatrai A, Harauy S, Piratae S, Khampoosa P, Kulsantiwong J, Donthaisong C, Chalokepanrat P, Viyanant V, Malone JB. Effects of Bayluscide on Bithynia siamensis goniomphalos, the first intermediate host of the human liver fluke, Opisthorchis viverrini, in laboratory and field trials. Parasitol Int 2012;61:52-55.
- 32. Tkatcheva V, Hyvärinen H, Kukkonen J, Ryzhkov LP, Holopainen IJ. Toxic effects of mining effluents on fish gills in a subarctic lake system in NW Russia. Ecotoxicol Environ Saf 2004;57:278-289.
- 33. Arellano JM, Storch V, Sarasquete C. Histological changes and copper accumulation in liver and gills of the Senegales sole, Solea senegalensis. Ecotoxicol Environ Saf 1999;44:62-72.
- 34. Kruatrachue M, Sumritdee C, Pokethitiyook P, Singhakaew S. Histopathological effects of contaminated sediments on golden apple snail (Pomacea canaliculata, Lamarck 1822). Bull Environ Contam Toxicol 2011;86:610-614.
- 35. Unlü E, Cengiz EI, Yildirim MZ, Otludil B, Unver O. Histopathological effects in tissues of snail Lymnaea stagnalis (Gastropoda, Pulmonata) exposed to sublethal concentration of Thiodan® and recovery after exposure. J Appl Toxicol 2005;25:459-463.
Fig. 1The representative tissue of control snails (A, B), snails exposed to camellia (C, D), snails exposed to mangosteen (E, F), and snails exposed to niclosamide (G, H). Hyperpigmentation of pigment cells, increasing numbers of lipid vacuoles, and atrophy of columnar muscle fibers were observed in camellia, mangosteen, and niclosamide-treated groups. Cm, columnar muscle fibers; Pt, protein cells; Pg, pigment cells; V, lipid vacuoles.

Fig. 2Representative gills with cilia of control snails (A, B), those exposed to camellia (C, D), those exposed to mangosteen (E, F), and those exposed to niclosamide (G, H). Camellia, mangosteen, and niclosamide-treated groups showed dilated interlamellar spaces and damaged cilia. Camellia group showed enlarged mucocyte cells with atypical nuclei. n, nuclei with dense heterochromatin; m, mucocytes; Ci, cilia; Ls, lamellar spaces.
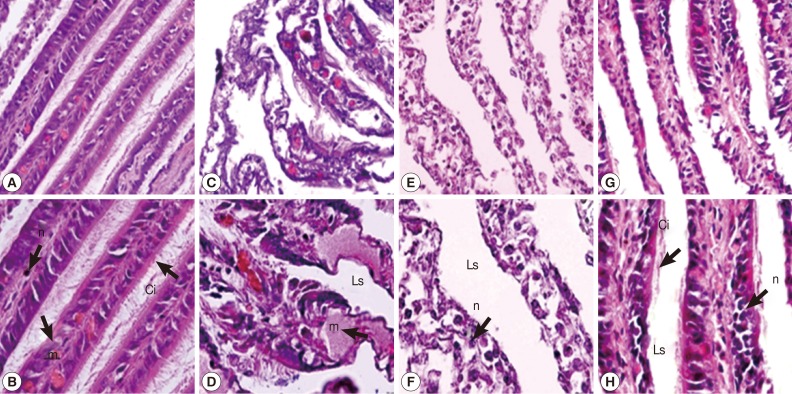
Fig. 3Representative digestive glands of control snails (A, B), those exposed to camellia (C, D), those exposed to mangosteen (E, F), and those exposed to niclosamide (G, H). Camellia, mangosteen, and niclosamide-treated groups showed a small number of calcium cells, dilation of the digestive gland, and large hemolymphatic spaces compared with the control snails. Dc, digestive cells; C, calciferous cells; Bs, basophilic cells; H, hemolymphatic spaces between the tubules; Pt, protein cells.
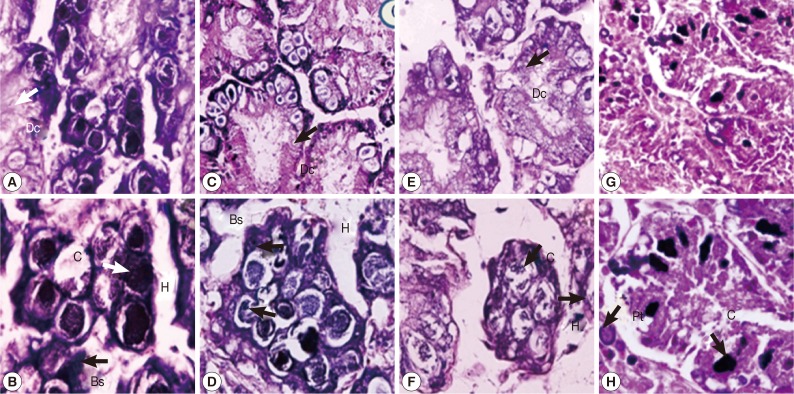
Fig. 4Epithelial lining of the digestive tract of control snails (A, B), those exposed to camellia (C, D), those exposed to mangosteen (E, F), and those exposed to niclosamide (G, H). Camellia and mangosteen-treated groups showed an increase of vacuolation in digestive cells and a decrease of ciliated cells compared with the control snails. Lu, lumen; Ci, cilia.

Fig. 5Representative epidermis of control snails (A), snails exposed to camellia (B), snails exposed to mangosteen (C), and snails exposed to niclosamide. The epidermis of normal snails showed regular surface, and camellia, mangosteen, and niclosamide-treated groups showed detachment of ciliated cells, enlarged lysosomal vacuoles, and an irregular epidermal surface. Ep, epidermis; Mv, microvilli; Lv, lysosomal vacuoles; Mu, mucous cell.