Abstract
Angiostrongylus cantonensis is a parasitic nematode that needs to develop in different hosts in different larval stages. Freshwater snails, such as Pomacea canaliculata, are the intermediate host, and rats are the definitive host. Periodic shedding of the cuticle (moulting) is an important biological process for the survival and development of the parasite in the intermediate and definitive hosts. However, there are few studies on the cuticle alterations between different stages of this parasite. In this study, we observed the ultrastructural appearance and changes of the cuticle of the 2nd/3rd stage larvae (L2/L3) and the 3rd/4th stage larvae (L3/L4) using a scanning electron microscope. We also first divided L2/L3 into late L2 and early L3. The late L2 lacked alae, but possessed a pull-chain-like fissure. Irregular alignment of spherical particles on the cuticle were noted compared to the L3. Alae appeared in the early L3. The old cuticle turned into a thin film-like structure which adhered to the new cuticle, and spherical particles were seen regularly arranged on the surface of this structure. Regular rectangular cavities were found on the surface of L3/L4. The caudal structure of L3/L4 was much larger than that of L3, but caudal inflation, such as seen in L4, was not observed. These results are the first to reveal the ultrastructural changes of the cuticle of A. cantonensis before and after moulting of L2/L3 and L3/L4.
-
Key words: Angiostrongylus cantonensis, moulting larva, scanning electron microscopy
INTRODUCTION
Angiostrongylus cantonensis was first found in the pulmonary artery of rats in China [
1] and is the agent of human angiostrongyliasis [
2]. The first stage larvae (L1) of this nematode can infect a snail intermediate host (
Pomacea canaliculata) and develop to the third-stage larvae (L3) within about 3 weeks [
3]. Although the rat is the permissive definitive host, humans can accidentally get infected by eating undercooked freshwater snails containing L3 larvae [
4], which penetrate the host blood-brain barrier and move into the central nervous system [
5]. In the permissive host, another 3 weeks or so is required for the L3s to develop into young adults and migrate to the pulmonary artery, where they develop sexual maturity to lay eggs [
6]. In the
A. cantonensis life cycle, 2 moults are required for L1 to become infective stage L3 in the snail intermediate host, and another 3 moults are required to become adults in the final host [
3].
Studies on a free-living nematode,
Caenorhabditis elegans, revealed that the cuticle was synthesized during late embryogenesis, then shed and re-synthesized at each larval stage underneath the existing cuticle, which is subsequently shed by a moulting process [
7]. Overlying the nematode cuticle is the lipid-rich epicuticle that is then covered by the glycoprotein-rich negatively-charged surface coat [
8].This labile accessory layer is associated with immune evasion in several parasitic nematodes [
9].
Although A. cantonensis was first described in 1935, the biology of the worm is still not clearly understood. Since the cuticle plays such an important role in nematodes, we believe that changes associated with moulting of the cuticle is vital for the parasite. The purpose of this study is to observe the cuticular changes in A. cantonensis during the moulting from L2 to L3 (L2/L3), and from L3 to L4 (L3/L4) using scanning electron microscopy (SEM).
MATERIALS AND METHODS
Parasite preparation
The L2/L3 larvae of
A. cantonensis were collected by a modified method of Parsons and Grieve [
10]. The tissues of infected snails (
Pomacea canaliculata) were minced and digested in a pepsin-hydrochloric acid solution at 37℃ for 2 hr. L2/L3 Larvae were collected under a dissecting microscope.
Five BALB/c mice aged 6 weeks were supplied by the Center of Animal Experiments of Sun Yat-sen University, Guangzhou, China. The procedures involving animals were approved by the Animal Care and Use Committee of Sun Yat-sen University. Animals were raised in a room with air conditioning under a 12/12-hr light/dark cycle.
All the mice were infected with A. cantonensis L3 (20 per mouse) using a stomach tube fitted with 1 ml syringe. At day 5 post-infection, all the mice were sacrificed by cervical dislocation. The brains were removed from the cranial cavity and were teased into small pieces. L3/L4 were collected under a dissecting microscope.
SEM
All the harvested larvae were washed 3 times with 0.2 M PBS (pH 7.2) and fixed in 0.2 M PBS containing 2.5% glutaraldehyde at 4℃ for 24 hr prior to electron microscopic observations. Specimens were washed 3 times in pH 7.2 PBS and 6 times in cold distilled water to remove glutaraldehyde and dehydrated through an ascending series of ethanol (50-100%). Then, ethanol was replaced with acetone and isoamyl acetate, and the worms were dried in a Hitachi HCP-2 critical-point drying machine using a transitional medium of liquid carbon dioxide. The specimens containing 2 L2/L3, 5 L3, 1 L3/L4, and 5 L4 were mounted on aluminum stubs and coated with platinum in an ion-sputtering apparatus (Hitachi E-102, Tokyo, Japan), and were examined and photographed in a scanning electron microscope (Hitachi S-2500, Tokyo, Japan).
RESULTS
Surface ultrastructure of L2/L3 from the snail host
L2/L3 larvae of
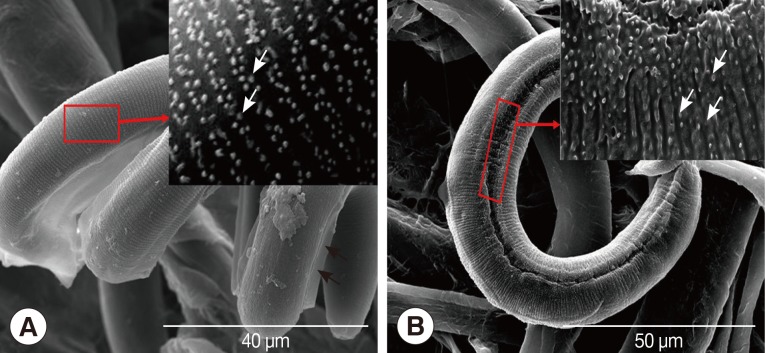
A. cantonensis were divided into late L2 and early L3 according to the time since recovery from the host snail. The SEM photograph of late L2 showed absence of alae, pull-chain-like fissure, and irregular alignment of the spherical particle structure on the cuticle of larvae comparing to L3 (
Fig. 1A, B). The SEM photograph of early L3 exhibited an appearance of alae. The old cuticle turned into a thin film-like structure with regularly arranged spherical particles on it (
Fig. 1A, B).
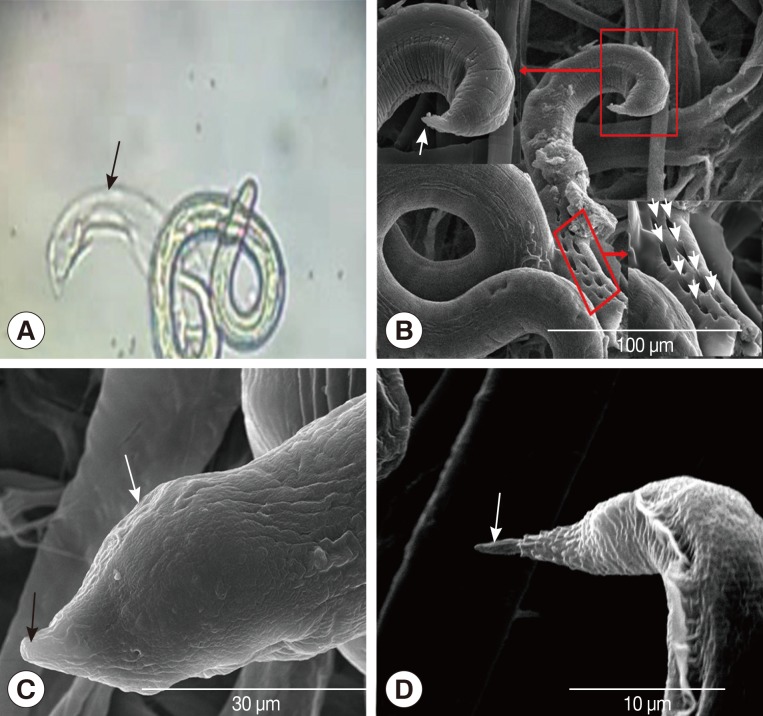
L3/L4 larvae were recovered from the brains of mice 5 days after L3 infection and were first thought to be mutilated specimens under a light microscope. However, by SEM observation, regular rectangular cavities were found on the surface of worm, and the phasmid of the worm was different from either L3 or L4 stages (
Fig. 2A, B). The caudal structure of the L3/L4 larvae was much larger than that of the L3 larvae, but caudal inflation was not observed (
Fig. 2C, D).
DISCUSSION
Both L1 and L3 were extraordinarily active in PBS pH 7.2 at 26℃, and the surfaces of these 2 kinds of larvae were smooth. However, L2/L3 were extraordinarily inactive at the same condition, with their bodies forming a C-shape, and the surfaces of larvae were rough. After the 2 layers of cuticles were synthesized and before they were moulted together, we designated this stage as L2/L3 larvae. Ding et al. [
11] reported SEM findings of various stages of this worm, and L2 was described to be difficult to distinguish the surface features whereas L3 and L4 were well described. However, the surface ultrastructure of moulting larvae was not reported.
According to the SEM pictures, we could divide L2/L3 larvae into 2 types, the early L3 and the late L2. The surface of late L2 presented a pull-chain-like fissure, with an irregularly arranged spherical particles on the cuticle, and alae were absent. In contrast, the surface of early L3 larvae exhibited alae, and the old cuticle turned into a thin film-like structure with spherical particles arranged regularly on it. Japanese researchers had cultured L1 to L3 in vitro, and reported that L2/L3 under molting retained 2 cuticles formed from L1 and L2 [
12].
Therefore, we propose a hypothesis about how L2/L3 moult their 2 layers of old cuticles. In late L2, the surface of the cuticle was deformed and turned into irregular spherical particles, and then the outside cuticle became a crevice-like structure. Gradually the larvae shed their outside cuticle like a jacket, thus the second layer of the cuticle inside became the outside cuticle. In early L3, the surface of the cuticle was deformed and turned into regular spherical particles which adhered to the new cuticle by a thin film-like structure. While the new cuticle was still growing, the old cuticle gradually degenerated around the helical worm body, like the pericarp shaved from an apple. The larvae recovered from the brain of mice 5 days after the infection were initially considered to be broken bodies of the larvae under a light microscope. However, further observation by SEM showed regular cavities on the surface of the larvae, and the tail of the larva was like neither the tail of L3 described by Ho et al. [
13] nor L4 described by Zeng et al. [
14]. We inferred from this that regular cavities were results of moulting, and the larva was at an intermediate stage of L3 and L4.
A. cantonensis, unlike the free living nematode C. elegans, completes moulting processes in the host tissue, and this phenomenon makes its moulting quite difficult. In summary, our SEM observations on the moulting of A. cantonensis established a morphological foundation for further research in moulting mechanisms of A. cantonensis.
Ministry of Science and Technology2010CB530004
National Nature Science Foundation81271855
National Nature Science Foundation81261160324
ACKNOWLEDGMENTS
This work was supported by the National Basic Research Program of China (grant no. 2010CB530004) and National Nature Science Foundation of China (grant no. 81271855, 81261160324).
References
- 1. Chen HT. Un nouveau nématode pulmonaire: Pulmonema cantonensis, n. g., n. sp. des rats de Canton. Ann Parasitol Hum Comp 1935;13:312-317.
- 2. Wang QP, Wu ZD, Wei J, Owen RL, Lun ZR. Human Angiostrongylus cantonensis: an update. Eur J Clin Microbiol Infect Dis 2012;31:389-395.
- 3. Liu HX, Zhang Y, Lv S, Hu L, Zhou XN. Establishment and observation of the life cycle of Angiostrongylus cantonensis in a laboratory setting. Chin J Pathogen Biol 2009;4:836-836. (in Chinese).
- 4. Wallace GD, Rosen L. Studies on eosinophilic meningitis. 2. Experimental infection of shrimp and crabs with Angiostrongylus cantonensis. Am J Epidemiol 1966;84:120-131.
- 5. Yoshimura K, Aiba H, Oya H, Fukuda Y. Angiostrongylus cantonensis: development following pulmonary arterial transfers into permissive and nonpermissive hosts. Exp Parasitol 1980;49:339-352.
- 6. Courdurier J, Guillon J, Malarde L, Laigret J, Desmoulins G, Schollhammer . Demonstration of the cycle of Angiostrongylus cantonensis in the laboratory: observations on this cycle and anatomo-pathology caused by this parasite in various laboratory animals. Bull Soc Pathol Exot Filiales 1964;57:1255-1262.
- 7. Singh R, Sulston J. Some observations on moulting in Caenorhabditis elegans. Nematologica 1978;24:63-71.
- 8. Gravato-Nobre MJ, Stroud D, O'Rourke D, Darby C, Hodgkin J. Glycosylation genes expressed in seam cells determine complex surface properties and bacterial adhesion to the cuticle of Caenorhabditis elegans. Genetics 2011;187:141-155.
- 9. Li BW, Rush AC, Mitreva M, Yin Y, Spiro D, Ghedin E, Weil GJ. Transcriptomes and pathways associated with infectivity, survival and immunogenicity in Brugia malayi L3. BMC Genomics 2009;10:267.
- 10. Parsons JC, Grieve RB. Effect of egg dosage and host genotype on liver trapping in murine larval toxocariasis. J Parasitol 1990;76:53-58.
- 11. Ding BL, Xu SE, Shen HX, Lu XJ, Zhang DP. Scanning electron microscopic observations on larvae and young adults of Angiostrongylus cantonensis. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi 1990;8:291-294. (in Chinese).
- 12. Hata H, Kojima S. Angiostrongylus cantonensis: in vitro cultivation from the first-stage to infective third-stage larvae. Exp Parasitol 1990;70:476-482.
- 13. Ho LY, Lawrencer A, Marietta V. Scanning Electron Microscope Studies of Adults, First- and Third-Stage Larvae of Angiostrongylus cantonensis (Nematoda : Metastrongyloidea). Proceed Helminthol Soc Wshington 1984;51:85-91.
- 14. Zeng X, Wang J, Wei J, Wu F, Fung F, Wu X, Sun X, Zheng H, Lv Z, Wu Z. Angiostrongylus cantonensis: tegumental and hypodermic alterations of the fourth-stage larvae following administration of tribendimidine in vivo and in vitro. Parasitol Res 2013;112:3035-3040.
Fig. 1SEM photographs of Angiostrongylus cantonensis early L3 and late L2. (A) Early L3 with alae (black arrows), a magnified view of the red frame area shows regularly arranged spherical particles (white arrows). (B) Picture of late L2 without alae, a magnified view of the red frame area shows irregularly arranged spherical particles (white arrows).

Fig. 2SEM and light microscopic photographs of Angiostrongylus cantonensis L3/L4. (A) A light microscopic photo of L3/L4, the black arrow shows molted part of the worm. (B) A SEM photograph of L3/L4, regular rectangular cavities (RRC) and L3/L4 tail in red flames are shown by white arrows at a greater magnification. (C) L3/L4 tail at greater magnification. L4 tail with obtuse phasmid (black arrow) and caudal inflation (white arrow). (D) L3 tail with a sharp phasmid is shown by white arrow.

Citations
Citations to this article as recorded by

- Water transmission potential of Angiostrongylus cantonensis: Larval viability and effectiveness of rainwater catchment sediment filters
Kathleen Howe, Lisa Kaluna, Alicia Lozano, Bruce Torres Fischer, Yaeko Tagami, Robert McHugh, Susan Jarvi, Matty Knight
PLOS ONE.2019; 14(4): e0209813. CrossRef - The genetic basis of adaptive evolution in parasitic environment from the Angiostrongylus cantonensis genome
Lian Xu, Meng Xu, Xi Sun, Junyang Xu, Xin Zeng, Dai Shan, Dongjuan Yuan, Ping He, Weiming He, Yulan Yang, Shiqi Luo, Jie Wei, Xiaoying Wu, Zhen Liu, Xiaomin Xu, Zhensheng Dong, Langui Song, Beibei Zhang, Zilong Yu, Lifu Wang, Chi Zhang, Xiaodong Fang, Qia
PLOS Neglected Tropical Diseases.2019; 13(11): e0007846. CrossRef - Molluscicidal activity and mechanism of toxicity of a novel salicylanilide ester derivative against Biomphalaria species
Ping He, Weisi Wang, Benjamin Sanogo, Xin Zeng, Xi Sun, Zhiyue Lv, Dongjuan Yuan, Liping Duan, Zhongdao Wu
Parasites & Vectors.2017;[Epub] CrossRef - Angiostrongylus cantonensis: a review of its distribution, molecular biology and clinical significance as a human pathogen
JOEL BARRATT, DOUGLAS CHAN, INDY SANDARADURA, RICHARD MALIK, DEREK SPIELMAN, ROGAN LEE, DEBORAH MARRIOTT, JOHN HARKNESS, JOHN ELLIS, DAMIEN STARK
Parasitology.2016; 143(9): 1087. CrossRef