Abstract
A field applicable diagnostic technique, the dipstick assay, was evaluated for its sensitivity and specificity in diagnosing human Schistosoma mansoni infection. A monoclonal antibody (mAb) against S. mansoni adult worm tegumental antigen (AWTA) was employed in dipstick and sandwich ELISA for detection of circulating schistosome antigen (CSA) in both serum and urine samples. Based on clinical and parasitological examinations, 60 S. mansoni-infected patients, 30 patients infected with parasites other than schistosomiasis, and 30 uninfected healthy individuals were selected. The sensitivity and specificity of dipstick assay in urine samples were 86.7% and 90.0%, respectively, compared to 90.0% sensitivity and 91.7% specificity of sandwich ELISA. In serum samples, the sensitivity and specificity were 88.3% and 91.7% for dipstick assay vs. 91.7% and 95.0% for sandwich ELISA, respectively. The diagnostic efficacy of dipstick assay in urine and serum samples was 88.3% and 90.0%, while it was 90.8% and 93.3% for sandwich ELISA, respectively. The diagnostic indices of dipstick assay and ELISA either in serum or in urine were statistically comparable (P>0.05). In conclusion, the dipstick assay offers an alternative simple, rapid, non-invasive technique in detecting CSA or complement to stool examinations especially in field studies.
-
Key words: Schistosoma mansoni, monoclonal antibody, dipstick assay, sandwich ELISA
INTRODUCTION
Parasitological examination of schistosome eggs in stool or urine is the gold standard in diagnosing the parasitic infection [
1-
4]. However, it is of limited value because of inadequate sensitivity especially in light infections, <50 eggs/g feces for
Schistosoma mansoni and <50 eggs/10 ml urine for
Schistosoma haematobium [
2,
3]. By time, antibody and antigen detection assays have been developed that led to an increase in sensitivity and specificity of these assays, especially the latter [
5]. Consequently, many tests have been developed to detect different circulating schistosome antigens (CSA) in urine or serum samples of infected hosts, e.g., sandwich ELISA [
6-
8], dot-ELISA [
9], latex agglutination test [
10], and dipstick assay [
11-
15], using either polyclonal or monoclonal antibodies (mAbs) [
16-
20].
Interestingly, only the urinary schistosomiasis dipstick has successfully been adopted for field use and was found to be highly sensitive and specific [
21], leading many researchers to recommend dipsticks for launching and monitoring mass treatment for
S. haematobium [
22]. So, this study aimed to develop a mAb-based dipstick test as a simple, rapid, and field applicable screening test for CSA in urine and serum samples of human schistosomiasis mansoni.
MATERIALS AND METHODS
Clinical examination
All patients were subjected to complete history taking and thorough clinical examinations. Recto-sigmoidoscopy with rectal snip was mainly performed for cases who had histories suggestive of schistosomiasis, e.g., bleeding from the rectum with repeatedly negative excreta. It was also performed for most parasitic cases to exclude concomitant schistosomal infection especially for those with history of schistosomiasis or living in an endemic area (farmers). Abdominal ultrasound examination was performed for all cases.
Parasitological examinations
Urine analysis was performed for all study groups using the sedimentation method [
23], and patients harboring
S. haematobium eggs in their excreta or having mixed parasitic infections were excluded from this study. Stool examination was performed using the merthiolate-iodine-formaldehyde-concentration (MIFC) method for detection of all helminth eggs and protozoal cysts [
24]. Subjects were divided into 3 groups,
S. mansoni patients (n=60), other parasite infected group (n=30), including
Fasciola gigantica (n=10),
Ancylostoma (n=10), and
Hymenolepis nana (n=10), and parasite-free healthy individuals (n=30).
S. mansoni eggs were quantified in stool samples on 3 consecutive days by the modified Kato's thick smear technique [
25]. The mean number of eggs was calculated. Blood and urine samples were collected from all cases, and sera were separated. Both sera and urine samples were aliquoted and kept at -70℃ until used.
Viable
S. mansoni adult worms were purchased from the Schistosome Biological Supply Program at Theodor Bilharz Research Institute, Giza, Egypt. The
S. mansoni AWTA (Sm AWTA) was prepared from living worms according to the method of Oaks et al. [
26].
The mAb (12D/10F) used in this study was produced by fusion of splenocytes of BALB/c mice immunized with Sm AWTA and BALB/c myeloma cell line (P3x63Ag.8) according to Galfre and Milstein [
27]. An IgM MAb (12D/10F) was found to be strongly reactive with
S. mansoni AWTA,
S. mansoni soluble egg antigen (SEA), and
S. haematobium SEA but not reactive with
Fasciola hepatica or
Echinococcus granulosus antigens as shown by sandwich ELISA. Also, it recognized
S. mansoni AWTA antigen with repetitive epitope at 50, 60, and 65 kDa bands as shown by immunoblotting which proved to be proteoglycan in nature [
10,
28].
Being reactive with a repetitive epitope, IgM mAb (12D/10F) was employed as antigen capturing and detecting antibody in sandwich ELISA according to Mohamed et al. [
28]. Labeling of IgM MAb with horseradish peroxidase was performed by periodate method according to Nakane and Kawaoi [
29]. After several optimization trials, sandwich ELISA was performed as originally described by Engvall and Perlmann [
30] and modified by Mohamed et al. [
28] and Ibrahim et al. [
10].
The mAb-dipstick assay described previously by van Etten et al. [
31] was performed as follows: Briefly, nitrocellulose (NC) membrane was coated with purified IgM mAb (200 µl of 1.25 µg/ml of 0.1 M carbonate buffer, pH 9.6) and then incubated overnight into the Bio-Rad-Slot apparatus (Bio-Rad, Hercules, California, USA) at 4℃. The NC membrane was washed with PBS/Tween (PBS/T) 4 times (1 min/wash), then with PBS 2 times (1 min/wash), and blocked with 2.5% fetal calf serum (Sigma, St. Louis, Missouri, USA) diluted in PBS/T, pH 7.4, for 30 min with shaking at room temperature (RT) and washed as previously mentioned. The NC membrane was dried and cut into 2 mm strips and supported into cassettes. The strips were incubated with 500 µl sera (diluted in blocking buffer 1:2) and urine samples (1 ml undiluted) for 30 min at RT with shaking, then washed with PBS/T for 4 times (1 min/wash). The strips were incubated with horseradish mAb peroxidase-conjugated IgM (10 µg/ml of PBS/Tween, 1 ml/strip) with shaking at RT for 30 min, then washed with PBS/T as previously mentioned. The substrate 3,3-diaminobenzidine (Sigma) was added (1 ml/strip) for 2 min at RT with shaking. The enzyme reaction was stopped by washing the strips 3 times with distilled water, then left to dry at RT. The detection of a brown band on the NC membrane meant positive serum or urine samples, while negative samples showed no color.
Data were expressed as mean±SD or number (%). Comparison between categorical data was performed using the chi-square test. Standard diagnostic indices, including sensitivity, specificity, and diagnostic efficacy were calculated as described by Galen [
32].
P-values less than or equal to 0.05 were considered significant. SPSS computer program (version 13, Windows, Chicago, Illinois, USA) was used for data analysis.
RESULTS
History taking of schistosomal cases showed that out of the 60
S. mansoni-infected patients, 50 (83.3%) had the history of exposure to canal water, 35 (58.3%) had the history of previous diagnosis of schistosomiasis, and 42 (70%) received anti-bilharzial treatment whether they were sure or not of the diagnosis. Clinical examination showed 28 (46.7%) patients with no palpable liver or spleen, 9 (15%) patients had hepatomegaly, 16 (26.7%) had hepatosplenomegaly, and 13 (21.7%) patients had shrunken liver and splenomegaly. The ultrasound findings as regards the liver showed that 37 (61.7%) patients were normal, 13 (21.7%) had periportal fibrosis, and 15 had cirrhotic patterns. Other sonographic findings were 30 (50%) with splenomegaly, 25 (41.7%) with portal vein dilatation, and 6 (10%) having ascites (
Table 1).
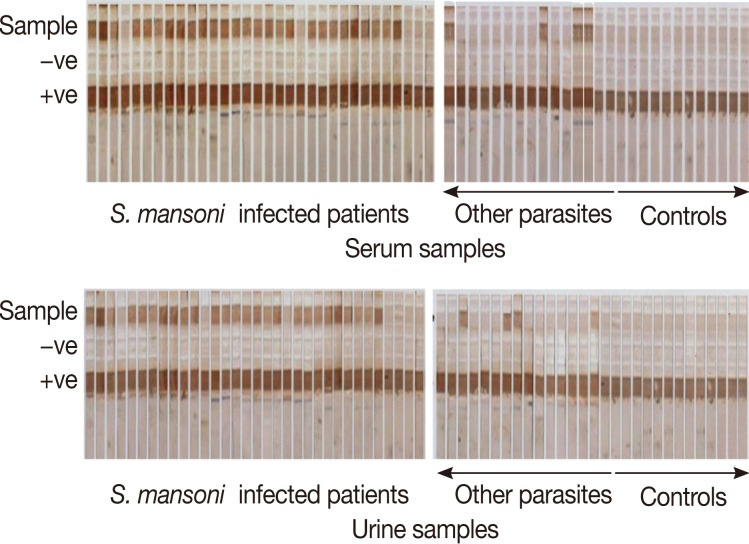
The dipstick assay was positive in urine samples in 52 out of 60
S. mansoni-infected patients giving 86.7% sensitivity, while in the serum, the dipstick assay was positive in 53 out of 60 Schistosoma-infected patients, and the sensitivity of the assay was 88.3% (
Fig. 1). Patients who showed false negative results in urine and serum samples were among the light infection subgroup, and the mean number of eggs in their stool/g was 19.6±6.7 and 23.5±7.2, respectively. The specificity of the assay was determined as the sum of results of the negative control group and other parasite infected group. All the 30 negative controls were dipstick negative in both urine and serum samples while 6 patients out of 30 other parasite infected group showed positive dipstick in urine and 5 patients in serum; they were considered as false positives. The specificity of the assay in urine and serum, therefore, was determined as 90% and 91.7%, respectively. The diagnostic efficacy of the assay was 88.3% and 90.8%, respectively (
Table 2).
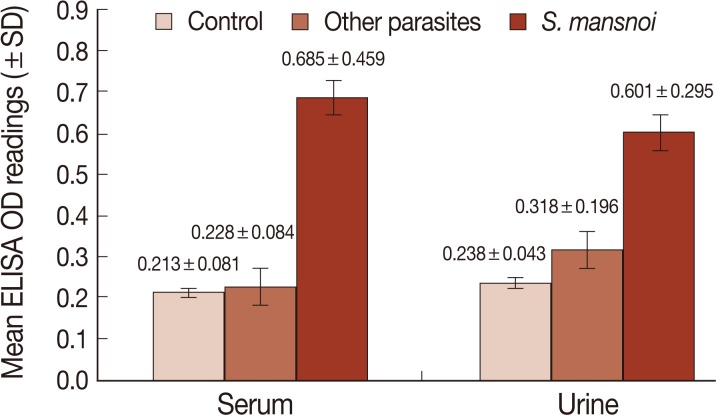
CSA in urine and serum of different studied groups were also measured by sandwich ELISA. The cut-off value was calculated as the mean optical density (OD) readings (at 492 nm) of negative controls and 2 SD of the mean. The OD readings equal to or less than the cut-off value were considered negative while those readings greater than the cut-off value were considered positive. In urine, the cut-off was 0.323, and ELISA was positive in 54 out of 60 schistosomiasis patients, and the sensitivity of the assay was 90%. In serum, the cut-off was 0.375. and ELISA was positive in 55 out of 60 schistosomiasis patients, and the sensitivity of the assay was 91.7% (
Fig. 2). Also, the 6 and 5 patients that showed false negative results in urine and serum were among the light infection subgroup. All the 30 negative controls were ELISA negative while 5 and 3 patients in other parasite infected group showed positive ELISA in urine and sera, respectively, and were considered as false positives. The specificity of the assay was 91.7% and 95%, respectively. The diagnostic efficacy of the assay was 90.8% and 93.3%, respectively (
Table 2). There was no statistically significant difference between the diagnostic indices of the dipstick assay and ELISA in serum or urine samples and at the same time between serum and urine within each of the techniques (
P>0.05).
DISCUSSION
In Egypt, a national control program against schistosomiasis was started from 1993 to 2006 leading to a gradual decrease in the prevalence of the 2 schistosome species (1.9% for
S. mansoni and 1.1% for
S. hematobium) [
33]. Since then, schistosomiasis is considered as a neglected disease [
33]. By time, attention paid to schistosomiasis has been reduced leading to an increase of its prevalence again specially in rural areas reaching to 10% [
34,
35, personal observations].
Nowadays, the use of ELISA for detection of schistosomal soluble egg antigen is recommended by many authors as a promising complementary field-applicable method for monitoring treatment and infection dynamics in endemic areas [
19,
36,
37]. This is an alternative to traditional parasitological approaches which became more fallible as both the infection prevalence and intensity diminished through control. Though ELISA had high sensitivity and specificity, yet it needs well-trained personnels and equipments and takes time to get results. Consequently, decision makers urged the need for simple, rapid, accurate, field applicable, and non-invasive alternative diagnostic approaches for schistosomiasis which led to the development of a dipstick test especially in highly endemic areas [
21].
The present study was carried out to detect CSA in both urine and serum samples of
S. mansoni-infected patients as well as patients infected with parasites other than
Schistosoma and healthy individual groups using a simple mAb-based dipstick test and comparing its results with mAb-based sandwich ELISA. The sensitivity of the dipstick assay in urine and serum samples was 86.7% and 88.3% compared to 90% and 91.7% by sandwich ELISA, respectively. The specificity of the dipstick assay was 90% and 91.7% for CSA assay in urine and sera, respectively, vs. 9.17% and 95% by sandwich ELISA. It is apparent that the sensitivity, specificity, and diagnostic efficacy of the dipstick assay in urine and serum samples was slightly lower than that detected by sandwich ELISA and at the same time it was slightly lower in urine than that reported in serum samples. Several researchers developed a commercial urine dipstick test for detecting circulating cathodic antigen (CCA) mainly against
S. haematobium infection [
15;
38] and used it in detecting
S. mansoni infection. The reported sensitivity of these dipstick assays ranged from 80% to 97.5% against
S. mansoni infection, while it gave contradictory results against
S. haematobium. For example, Hendawy et al. [
14] evaluated the use of mAb-based dipstick as a qualitative assay for rapid diagnosis of
S. mansoni. The sensitivity and specificity of the dipstick assay was 97.5% and 96.2%, respectively. Also, Stothard et al. [
15] reported 83% sensitivity and 81% specificity of the dipstick for detecting CCA of
S. mansoni. Again, Stothard et al. [
37] confirmed the same results and reported 95% sensitivity and 80% specificity for
S. mansoni and reported unsatisfactory results for
S. haematobium, while it was 89% using SEA-ELISA assay. Also, Ashton et al. [
39] reported that the sensitivity and specificity of the dipstick was 89.1% and 74.2% for
S. mansoni vs. 36.8% and 78.9% for
S. haematobium. On the other hand, Bosompem et al. [
21] developed a mAb-based dipstick assay against
S. haematobium in endemic areas and reported 99.1% sensitivity and 98.3% specificity. Also, in 2004, they reported 98.8% sensitivity and 53.6% specificity [
40]. The slight increase in the diagnostic efficacy of the dipstick in our study compared to others could be due to the target antigen used and the nature of antibody directed against different Schistosoma species, raising the necessity to evaluate the efficacy of this technique against
S. haematobium.
Detection of CSA in urine instead of serum offers an alternative non-invasive way, especially in large control programs in endemic areas where the process is cumbersome and tedious for schoolchildren who are the main targets for such control.
In conclusion, further studies are recommended to improve the diagnostic efficacy and to decrease the cost of the dipstick, e.g., using a nanoparticle colloidal dye conjugated with the mAb that increases their binding surface area and decreases their hydrophobicity which in turn block the binding sites and consequently decrease the cross-reaction of non-specific antigens.
ACKNOWLEDGMENT
This work was supported by internal project No. 65 D at Theodor Bilharz Research Institute, Giza, Egypt.
References
- 1. Montresor A, Crompton DW, Gyorkos TW, Savioli L. Helminth control in school age children: a guide for managers of control programmes. 2002, Geneva, Switzerland. World Health Organization.
- 2. WHO. Report of the WHO informal consultation on schistosomiasis in low transmission areas: control strategies and criteria for elimination. WHO/CDS/CPE/SIP/2001.1. 2000, London, UK. World Health Organization.
- 3. WHO. Prevention and control of schistosomiasis and soil-transmitted helminthiasis: report of a WHO Expert Committee. WHO Tech Rep Series. 2002, Vol. 912:Geneva, Switzerland. World Health Organization.
- 4. Katz N, Coelho PMZ. Clinical therapy of schistosomiasis mansoni: the Brazilian contribution. Acta Trop 2008;108:72-78.
- 5. Qian ZL, Deelder AM. Schistosoma japonicum: immunological response to circulating polysaccharide antigens in rabbits with a light infection. Exp Parasitol 1983;55:394-403.
- 6. Shaker ZA, Kaddah MA, Hanallah SB, El-Khodary MI. Production of monoclonal antibodies against target schistosomal antigen secreted in the urine of Schistosoma mansoni-infected patients. Int J Parasitol 1998;28:1893-1901.
- 7. Hanallah SB, Mohamed SH, Maher KM, Amer NM, Sayed HA, Demerdash ZA, Shaker ZA. A monoclonal antibody against tegument of adult S. mansoni worms detects active schistosomal infection. Kasr El Aini Med J 2003;9:209-223.
- 8. Salah F, El Bassiouny A, Rabie I, Demerdash Z, Roshdy M, Shaker Z. Human schistosomiasis haematobium: effective diagnosis of active infection using a pair of monoclonal antibodies against soluble egg antigen. Parasitol Res 2006;99:528-533.
- 9. Mahfouz AM, Rabee I, El Amir A. Evaluation of different immunological techniques for diagnosis of schistosomiasis haematobium in Egypt. Biotechnology 2012;11:10-19.
- 10. Ibrahim RB, Mohamed SH, Demerdash ZA, Diab TM, Maher K, Safwat W, Shaker ZA. Anti-S. mansoni mAb-based latex agglutination: a reliable field applicable immunodiagnostic test for screening of active human schistosomiasis. J Am Sci 2010;6:19-27.
- 11. Gezahegan T. Development of a dipstick method for mosquito blood-meal identification and comparison with ELISA for potential field use. Med Sei 1989;42:593-600.
- 12. Stott DI. Immunoblotting and dot blotting. J Immunol Methods 1989;119:153-187.
- 13. Van Dam GJ, Wichers JH, Ferreira TM, Ghati D, van Amerongen A, Deelder AM. Diagnosis of schistosomiasis by reagent strip test for detection of circulating cathodic antigen. J Clin Microbiol 2004;42:5458-5461.
- 14. Hendawy MA, Mohamed SH, Ibrahim RA. Nitrocellulose cassettes (NCC) as a field applicable technique for rapid and effective diagnosis of schistosomiasis and fascioliasis. New Egypt J Med 2006;35:86-92.
- 15. Stothard JR, Kabatereine NB, Tukahebwa EM, Kazibwe F, Rollinson D, Mathieson W, Webster JP, Fenwick A. Use of circulating cathodic antigen (CCA) dipsticks for detection of intestinal and urinary schistosomiasis. Acta Trop 2006;97:219-228.
- 16. Deelder AM, De Jonge N, Boerman OC, Fillié YE, Hilberath GW, Rotmans JP, Gerritse MJ, Schut DW. Sensitive determination of circulating anodic antigen in Schistosoma mansoni infected individuals by an enzyme-linked immunosorbent assay using monoclonal antibodies. Am J Trop Med Hyg 1989;40:268-272.
- 17. Van Lieshout L, De Jonge N, Mansour MM, Bassily S, Krijger FW, Deelder AM. Circulating cathodic antigen levels in serum and urine of schistosomiasis patients before and after chemotherapy with praziquantel. Trans R Soc Trop Med Hyg 1993;87:311-312.
- 18. Nibbeling HA, Van Lieshout L, Deelder AM. Levels of circulating soluble egg antigen in urine of individuals infected with Schistosoma mansoni before and after treatment with praziquantel. Trans R Soc Trop Med Hyg 1998;92:675-677.
- 19. Mahmoud S, Demerdash ZA, Mohamed SH, Badawy AA, Mohamed SH, Shaker ZA, Ibrahim AM. Study of kinetics of circulating schistosome antigens in tissue and sera of infected mice using anti-Schistosoma mansoni soluble egg antigen monoclonal antibody. Egypt J Schistoso Infect Endem Dis 2002;24:1-17.
- 20. Cai YC, Guo J, Chen SH, Tian LG, Steinmann P, Chen MX, Li H, Ai L, Chen JX. Chicken egg yolk antibodies (IgY) for detecting circulating antigens of Schistosoma japonicum. Parasitol Int 2012;61:385-390.
- 21. Bosompem KM, Ayi I, Anyan WK, Nkrumah FK, Kojima S. Limited field evaluation of a rapid monoclonal antibody-based dipstick assay for urinary schistosomiasis. Hybridoma 1996;15:443-447.
- 22. Emukah E, Gutman J, Eguagie J, Miri ES, Yinkore P, Okocha N, Jibunor V, Nebe O, Nwoye AI, Richards FO. Heme dipsticks are useful in monitoring the impact of praziquantel treatment on Schistosoma haematobium in sentinel communities of Delta State, Nigeria. Acta Trop 2012;122:126-131.
- 23. Peters PA, Mahmoud AA, Warren KS, Ouma JH, Arap Siongok TK. Field studies of a rapid, accurate means of quantifying Schistosoma haematobium eggs in urine samples. Bull WHO 1976;54:159-162.
- 24. Blagg W, Mansour NS, Khalaf GL. A new concentration technique for the demonstration of protozoa and helminthic eggs in feces. Am J Trop Med Hyg 1955;4:23.
- 25. Arap Siongok TK, Mahmoud AA, Ouma JH, Warren KS, Muller AS, Handa AK, Houser HB. Morbidity in schistosomiasis mansoni in relation to intensity of infection: study of a community in Machakos, Kenya. Am J Trop Med Hyg 1976;25:273-284.
- 26. Oaks JA, Cain A, Mower DA, Raj RK. Distribution and removal of the tegument from Schistosoma mansoni with triton X-100. J Parasitol 1981;67:761-774.
- 27. Galfre G, Milstein C. Properties of monoclonal antibodies: strategies and procedures. Meth Enzymol 1981;73:3.
- 28. Mohamed S, Diab TM, Safwat W, Demerdash Z, Shaker Z. An effective anti-S. mansoni monoclonal antibody for detection of human antigenaemia and antigenurea. Egypt J Med Sci 2007;12:25-33.
- 29. Nakane PK, Kawaoi A. Peroxidase-labeled antibody. A new method of conjugation. J Histochem Cytochem 1974;22:1084-1091.
- 30. Engvall E, Perlman P. Enzyme-linked immunosorbent assay (EL1SA). Quantitative assay of immunuglobulin G. J Immunochem 1971;8:871-874.
- 31. van Etten L, Folman CC, Eggelte TA, Kremsner PG, Deelder AM. Rapid diagnosis of schistosomiasis by antigen detection in urine with a reagent strip. J Clin Microbiol 1994;32:2404-2406.
- 32. Galen RS. Predictive values and efficiency of laboratory testing. Pediatr Clin North Am 1980;27:861-869.
- 33. WHO. Gearing up to eliminate schistosomiasis with increased donation of praziquantel from Merck KGaA. 2012, Geneva, Switzerland. World Health Organization.
- 34. Utzinger J, Raso G, Brooker S, De Savigny D, Tanner M, Ornbjerg N, Singer BH, N'goran EK. Schistosomiasis and neglected tropical diseases: towards integrated and sustainable control and a word of caution. Parasitology 2009;136:1859-1874.
- 35. Allam AF. Diagnosis of schistosomiasis in low endemic areas. In Tech Open Book 2012;1:1-10. (www.intechopen.com/download/pdf/pdfs)
- 36. Salah F, Demerdash Z, Shaker Z, El Bassiouny A, El Attar G, Ismail S, Abadir N, Saad El Din A, Mansour M. A monoclonal antibody against Schistosoma haematobium soluble egg antigen(s): efficacy for diagnosis and monitoring of cure of Schistosoma haematobium infection. Parasitol Res 2000;86:74-80.
- 37. Stothard JR, Sousa-Figueiredo JC, Standley C, Van Dam GJ, Knopp S, Utzinger J, Ameri H, Khamis AN, Khamis IS, Deelder AM, Mohammed KA, Rollinson D. An evaluation of urine-CCA strip test and fingerprick blood SEA-ELISA for detection of urinary schistosomiasis in schoolchildren in Zanzibar. Acta Trop 2009;111:64-70.
- 38. Bosompem KM, Ayi I, Anyan WK, Arishima T, Nkrumah FK, Kojima S. A monoclonal antibody-based dipstick assay for diagnosis of urinary schistosomiasis. Trans R Soc Trop Med Hyg 1997;91:554-556.
- 39. Ashton RA, Stewart BT, Petty N, Lado M, Finn T, Brooker S, Kolaczinski JH. Accuracy of circulating cathodic antigen tests for rapid mapping of Schistosoma mansoni and S. haematobium infections in Southern Sudan. Trop Med Int Health 2011;16:1099-1103.
- 40. Bosompem KM, Owusu O, Okanla EO, Kojima S. Applicability of a monoclonal antibody-based dipstick in diagnosis of urinary schistosomiasis in the Central Region of Ghana. Trop Med Int Health 2004;9:991-996.
Fig. 1Detection of CSA in serum and urine samples of different study groups by the dipstick assay. Dipsticks were dotted with the adult worm tegumental antigen (AWTA) as the positive control and PBS as the negative control.
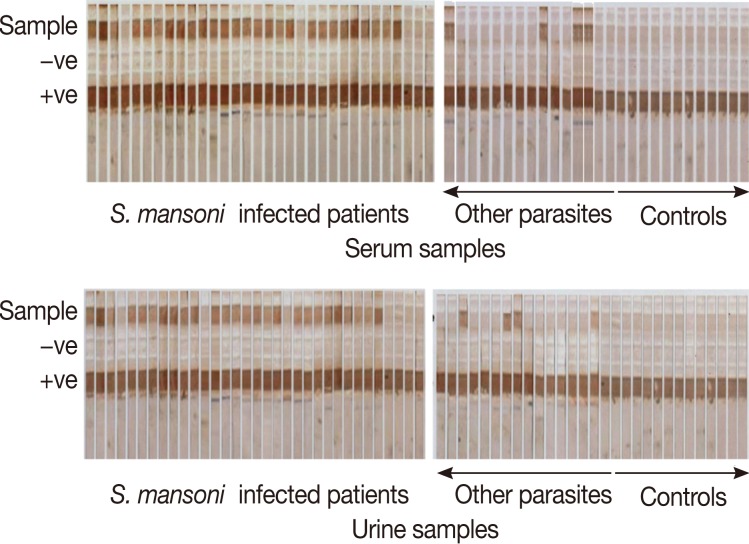
Fig. 2Mean level of CSA (±SD) in sera and urine samples of different study groups measured by sandwich ELISA (OD reading at 492 nm).
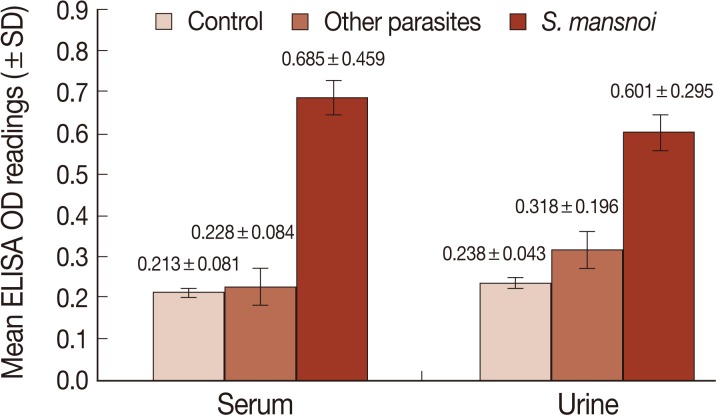
Table 1.Clinical and sonographic findings of S. mansoni and other parasite-infected groups
Table 1.
|
No. of cases (%)
|
|
S. mansoni (n = 60) |
Other parasites (n = 30) |
|
History of exposure to canal water |
50 (83.3) |
10 (33.3) |
|
Previous history of schistosome infection |
35 (58.3) |
1 (3.3) |
|
Previous schistosome treatment |
42 (70.0) |
1 (3.3) |
|
Bleeding/rectum, tenesmus |
5 (8.3) |
2 (6.7) |
|
Clinical findings |
|
|
|
Normal |
28 (46.7) |
20 (66.7) |
|
Hepatomegaly |
9 (15.0) |
5 (16.7) |
|
Hepatospelnomegaly |
16 (26.7) |
4 (13.3) |
|
Shrunken liver & splenomegaly |
13 (21.7) |
1 (3.3) |
|
Sonographic findings (liver) |
|
|
|
Normal/periportal thickening/cirrhosis |
37/13/15 (61.7/21.7/50.0) |
20/7/3 (66.7/23.3/10.0) |
|
Portal vein dilatation |
25 (41.7) |
1 (3.3) |
|
Splenomegaly |
30 (50.0) |
2 (6.6) |
|
Ascites |
6 (10.0) |
0 (0.0) |
|
Kidney disease |
0 (0.0) |
0 (0.0) |
Table 2.Diagnostic indices (sensitivity, specificity, and diagnostic efficacy) of dipstick and ELISA in studied groups
Table 2.
|
Dipstick
|
ELISA
|
|
Urine |
Serum |
Urine |
Serum |
|
Sensitivity (%) |
86.7 |
88.3 |
90.0 |
91.7 |
|
Specificity (%) |
90.0 |
91.7 |
91.7 |
95.0 |
|
Diagnostic efficacy (%) |
88.3 |
90.0 |
90.8 |
93.3 |