Abstract
Recombinant antigenic proteins of Toxoplasma gondii are alternative source of antigens which are easily obtainable for serodiagnosis of toxoplasmosis. In this study, highly antigenic secretory organellar proteins, dense granular GRA2 and GRA3, rhoptrial ROP2, and micronemal MIC2, were analyzed by bioinformatics approach to express as water-soluble forms of antigenic domains. The transmembrane region and disorder tendency of 4 secretory proteins were predicted to clone the genes into pGEX-4T-1 vector. Recombinant plasmids were transformed into BL21 (DE3) pLysS E. coli, and GST fusion proteins were expressed with IPTG. As a result, GST fusion proteins with GRA225-105, GRA339-138, ROP2324-561, and MIC21-284 domains had respectively higher value of IgG avidity. The rGST-GRA225-105 and rGST-GRA339-138 were soluble, while rGST-ROP2324-561 and rGST-MIC21-284 were not. GRA231-71, intrinsically unstructured domain (IUD) of GRA2, was used as a linker to enhance the solubility. The rGST-GRA231-71-ROP2324-561, a chimeric protein, appeared to be soluble. Moreover, rGST-GRA231-71-MIC21-284 was also soluble and had higher IgG avidity comparing to rGST-MIC21-284. These 4 highly expressed and water-soluble recombinant antigenic proteins may be promising candidates to improve the serodiagnosis of toxoplasmosis in addition to the major surface antigen of SAG1.
-
Key words: Toxoplasma gondii, bioinformatics, intrinsically unstructured domain, GRA2, GRA3, ROP2, MIC2, antigenic domain, IgG avidity
INTRODUCTION
Toxoplasma gondii is an obligate intracellular protozoan parasite and an important zoonotic pathogen [
1]. It infects a broad range of warm blooded animals, including human beings, causing severe congenital defects, abortion, and neonatal complications [
2]. Acquired infection is also possible, of which almost of all are benign and asymptomatic but the infection proceeds to a chronic cyst formation stage in the central nervous system or in muscle. Sometimes retinochoroiditis and meningoencephalitis occur in newly infected and reactivated cases with the brain cyst [
3,
4].
For serodiagnosis of toxoplasmosis, lots of commercial serological kits are developed most of which are based on
Toxoplasma lysate antigens (TLA) [
5]. Recently, many studies showed that recombinant proteins of
T. gondii may be an alternative source of antigens due to producing safer diagnostic antigens with lower cost of production and purification [
6]. Three major advantages of the recombinant antigens for the diagnosis of
T. gondii infection are summarized [
5,
7] such that the composition of recombinant antigens is precisely known, the use of more than 1 defined antigen, and the method can be easily standardized. On the while, 2 disadvantages of using recombinant antigens are described as the problem of expression efficiency of the different antigens in
Escherichia coli [
8] and mis-folding of the recombinant antigens. It may not be very identical to native antigens that are produced by
T. gondii, since there are different ways in that the molecule is folded in
E. coli and
T. gondii [
9]. The importance of the folding process may affect the ability of antibody production against native antigen to recognize and bind to a defined recombinant antigen with the same affinity. Recombinant proteins fused with an intrinsically unstructured domain (IUD) of GRA2 enhanced diagnostic sensitivity, wherein the IUD is flexible and helps the proteins folded correctly to expose the antigenic domains [
10].
Antigens of
T. gondii are composed of surface antigens as well as several others from specific secretory organelles: micronemes, rhoptries, and dense granules [
11]. SAG1, the major surface antigen, is well studied and analyzed [
12]. GRA2 and GRA3, dense granular antigens, are well explored and summarized in several studies [
13,
14,
15]. ROP2, a rhoptry antigen, contains an ordered catalytic domain of kinase [
16]. MIC2, a micronemal protein, has no signal sequence peptides in N-terminal but has a transmembrane region in C-terminal [
17]. Many studies developed recombinant antigens with surface antigens and dense granule proteins, but micronemal and rhoptry proteins were merely chosen [
5]. In the present study, we analyzed GRA2, GRA3, ROP2, and MIC2 using bioinformatics approaches [
18,
19] to dissect the antigenic domain of each protein. The diagnostic value of the fragmented recombinant antigens was analyzed in an IgG avidity test. Four low molecular weight recombinant proteins including 2 chimeric proteins which have relatively higher value of IgG avidity are identified as highly yielded and water soluble.
MATERIALS AND METHODS
Parasites and sera
Tachyzoites of RH strain of
T. gondii were injected into BALB/c mice intraperitoneally, and peritoneal exudates were collected right after the death of mice with Dulbecco's Phosphate Buffered Saline (DPBS) at day 4. Fresh RH tachyzoites were washed in DPBS and collected after centrifugation as
Toxoplasma lysate antigen (TLA). Sera of patients of toxoplasmosis were tested positively by ELISA as in a previous study [
20].
Horseradish peroxidase (HRP)-conjugated anti-rabbit antibody and HRP-conjugated anti-human IgG antibody were purchased from Sigma Aldrich (St. Louis, Missouri, USA). Rabbit monoclonal GST antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, California, USA). The pGEX-4T-1 vector and BL21 (DE3) pLysS E. coli were from GE Healthcare (Little Chalfont, UK). RNA extraction kit and plasmid prep kit were from Gene All (Seoul, Korea). The cDNA synthesis kit was from Clontech Laboratories (Mountain View, California, USA). DH5α E. coli, PCR synthesis kit, T4 ligation kit and restriction enzyme digestion kit were from Enzynomics (Seoul, Korea). Primers were synthesized in Bioneer Corporation (Daejeon, Korea). Isopropyl β-D-1-thiogalactopyranoside (IPTG) and ampicillin were from Duchefa Biochemie (Haarlem, The Netherlands).
Bioinformatics analysis
Four secretory organellar
T. gondii proteins were chosen according to highly antigenic excretory/secretory proteins reported in a previous study [
21]. Intrinsically unstructured regions of those proteins were predicted by IUPred server (
http://iupred.enzim.hu/). Transmembrane regions of those proteins were predicted by DAS server (
http://www.sbc.su.se/~miklos/DAS/maindas.html). Based on the transmembrane regions and intrinsically unstructured regions of each protein, several sets of primers [
18,
19] were designed to subclone fragments of genes into pGEX-4T-1 vector, respectively.
Total RNA extraction and cDNA synthesis were done according to the manuals of manufacturers. Target fragments were amplified by PCR with designed primers (
Table 1). PCR products and pGEX-4T-1 vector were purified and digested by restriction enzymes. Digested fragments were ligated into the pGEX-4T-1 vector. After transformation, recombinant plasmids were amplified in DH5α.
The pGEX-4T-1 vector and recombinant plasmids were extracted from DH5α E. coli and transformed into BL21 (DE3) pLysS E. coli. GST and GST fusion proteins were induced at 0.5 mM IPTG. Pellets of E. coli after induction were sonicated in DPBS. The mixture of the sonication was regarded as the total lysates of E. coli expressing GST recombinant proteins. After centrifugation at 16,000 g, 4℃ for 10 min, the supernatant was saved as a soluble fraction and the precipitant as an insoluble fraction. Following western blot, the expression and solubility of GST fusion proteins were examined with rabbit monoclonal GST antibody.
Western blot analysis
Samples were dissolved by SDS-PAGE and transferred to a nitrocellulose (NC) membrane (Whatman GmbH, Dassel, Germany) by mini-protean Tetra system (BioRad, Hercules, California, USA). The membrane was incubated with 5% skim milk (Difco Laboratories, Detroit, Michigan, USA) in PBS with 0.5% Tween 20 (PBST) for 1hr. After washing with PBST, membranes were incubated with the 1st antibody in PBST with 5% skim milk at room temperature (RT) for 2 hr. It was then incubated with the 2nd antibody in PBST with 5% skim milk at RT for 2 hr. Signals were detected with ECL Western blotting kit (Millipore Co., Billerica, Massachusetts, USA).
Analysis of the antigenicity of recombinant proteins
Total lysates of E. coli and TLA were transferred to NC membrane and incubated with patient serum as the 1st antibody in western blot. Then, membranes were incubated with HRP-conjugated anti-human IgG antibody. Signals were detected with ECL western blotting kit.
RESULTS
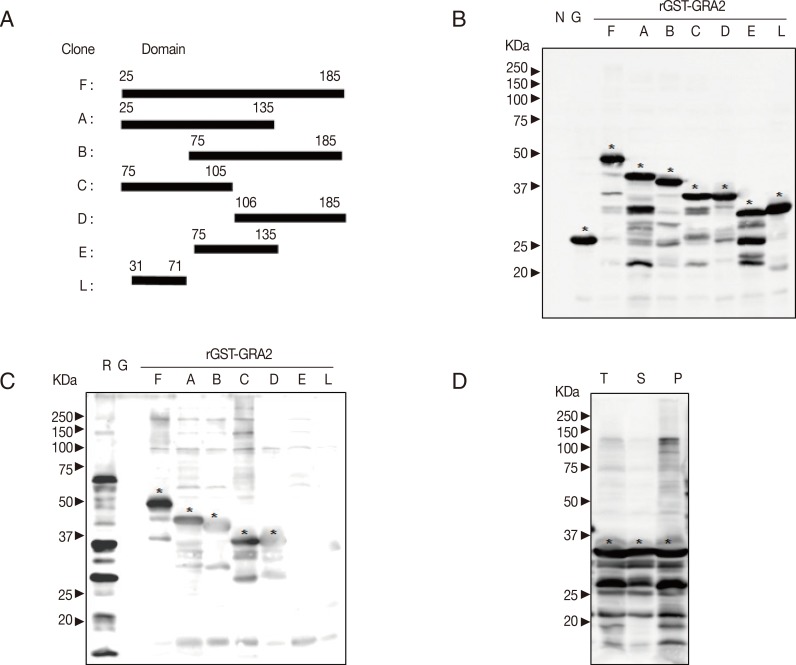
Construction and expression of GST-GRA2 fusion protein
The transmembrane regions and the intrinsically unstructured domains of GRA2 (GenBank no. HM014012.1) were predicted by DAS server and IUPred server [
18,
19]. The transmembrane region of GRA2 is in its N-terminal as a signal sequence. The GRA2 shows higher disorder tendency except the 36 amino acids of N-terminal containing signal sequence. Based on the analysis of the structure, 7 fragments of cDNA of GRA2 were subcloned into pGEX-4T-1 vector in
BamHI,
EcoRI, or
XhoI sites. Cloning domains and corresponding sequences of cDNA are shown in
Fig. 1A.
The pGEX-4T-1 and recombinant plasmids were transformed into BL21 (DE3) pLysS
E. coli. All recombinant proteins were induced at 30℃ with 0.5 mM IPTG. As shown in
Fig. 1B, rGST-GRA2 fusion proteins were well expressed. In
Fig. 1C, rGST-GRA2
25-185, rGST-GRA2
25-135, and rGST-GRA2
25-105 show stronger antigenicity, while rGST-GRA2
75-185 and rGST-GRA2
106-185 show weaker antigenicity. The solubility of rGST-GRA2
25-105 was tested as shown in
Fig. 1D; it was expressed at 30℃ with 0.5 mM IPTG as a soluble form.
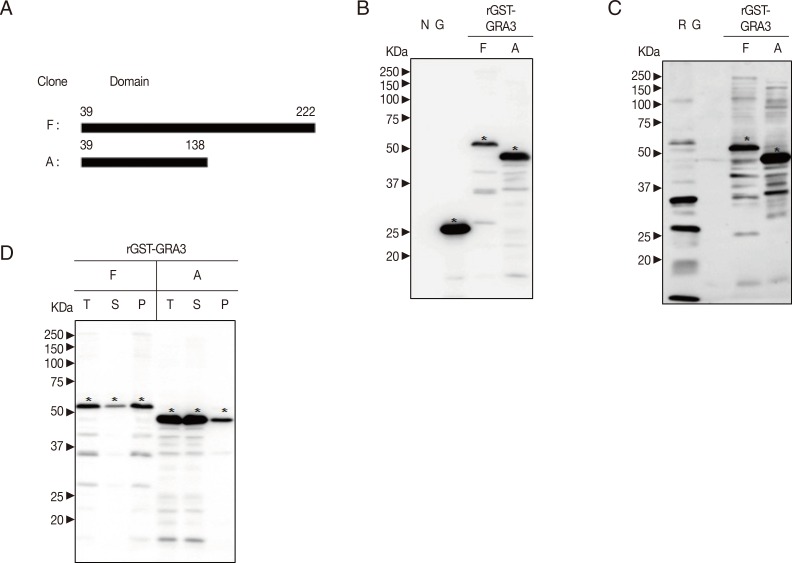
The main transmembrane region of GRA3 (GenBank no. EF552406.1) is in its N-terminal, and the middle of C-terminal of GRA3 shows relatively higher profile score. The whole fragment of GRA3 is predicted with lower disorder tendency comparing to GRA2. Based on the analysis of the structure, 2 fragments of cDNA of GRA3 were subcloned into pGEX-4T-1 vector in
EcoRI and
XhoI sites. Cloning domains and corresponding sequences of cDNA are shown in
Fig. 2A.
All recombinant proteins in BL21 (DE3) pLysS
E. coli were induced at 30℃, 0.5 mM IPTG. As shown in
Fig. 2B and D, rGST-GRA3
39-138 fusion protein was well expressed, but rGST-GRA3
39-222 was not. As shown in
Fig. 2C, rGST-GRA3
39-138 and rGST-GRA3
39-222 present equivalent antigenicity. The rGST-GRA3
39-222 is slightly soluble, and rGST-GRA3
39-138 is significantly soluble at 30℃, 0.5 mM IPTG in
Fig. 2D.
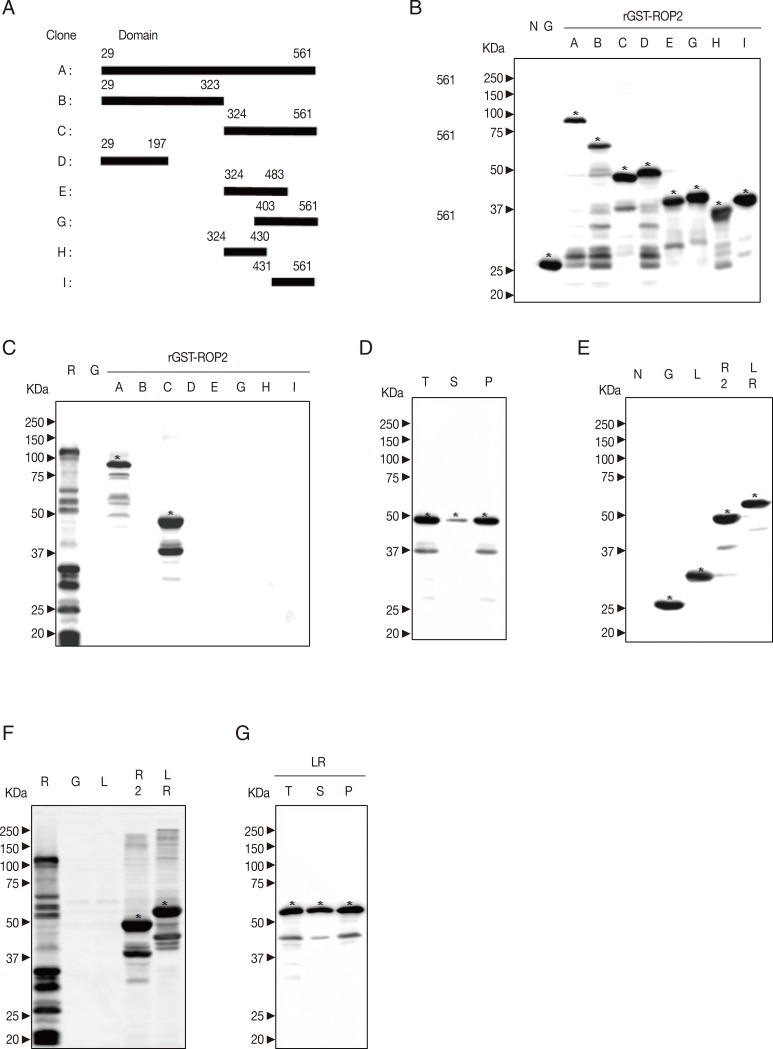
The main transmembrane region of ROP2 (GenBank no. Z36906.1) is in its N-terminal. The N-terminal sequence of ROP2 except peptide signal sequence is predicted to have higher disorder tendency, while the kinase domain of ROP2 shows lower disorder tendency. Based on these analyses, 8 fragments of cDNA of ROP2 were subcloned into pGEX-4T-1 vector in
EcoRI or
XhoI sites. Cloning domains and corresponding sequences of cDNA are shown in
Fig. 3A.
The pGEX-4T-1 and recombinant plasmids were transformed into BL21 (DE3) pLysS
E. coli. As shown in
Fig. 3B, rGST-ROP2 fusion proteins were well expressed at 30℃, 0.5 mM IPTG. In
Fig. 3C, rGST-ROP2
29-561 and rGST-ROP2
324-561 showed higher antigenicity. However, rGST-ROP2
324-561 is slightly soluble at 20℃ and 30℃, 0.5 mM IPTG in
Fig. 3D.
The domain of GRA2
31-71 is predicted with a low profile score and high disorder tendency to be chosen as a linker. The pGEX-4T-1/GRA2
31-71 and PCR product of ROP2
324-561 were digested by
EcoRI restriction digestion enzyme. Two linear DNA were ligated and transformed into BL21 (DE3) pLysS
E. coli. In
Fig. 3E and F, rGST-GRA2
31-71-ROP2
324-561 protein was well expressed and possessed same antigenicity as rGST-ROP2
324-561. However, rGST-GRA2
31-71-ROP2
324-561 protein was soluble at 20℃, 0.5 mM IPTG (
Fig. 3G).
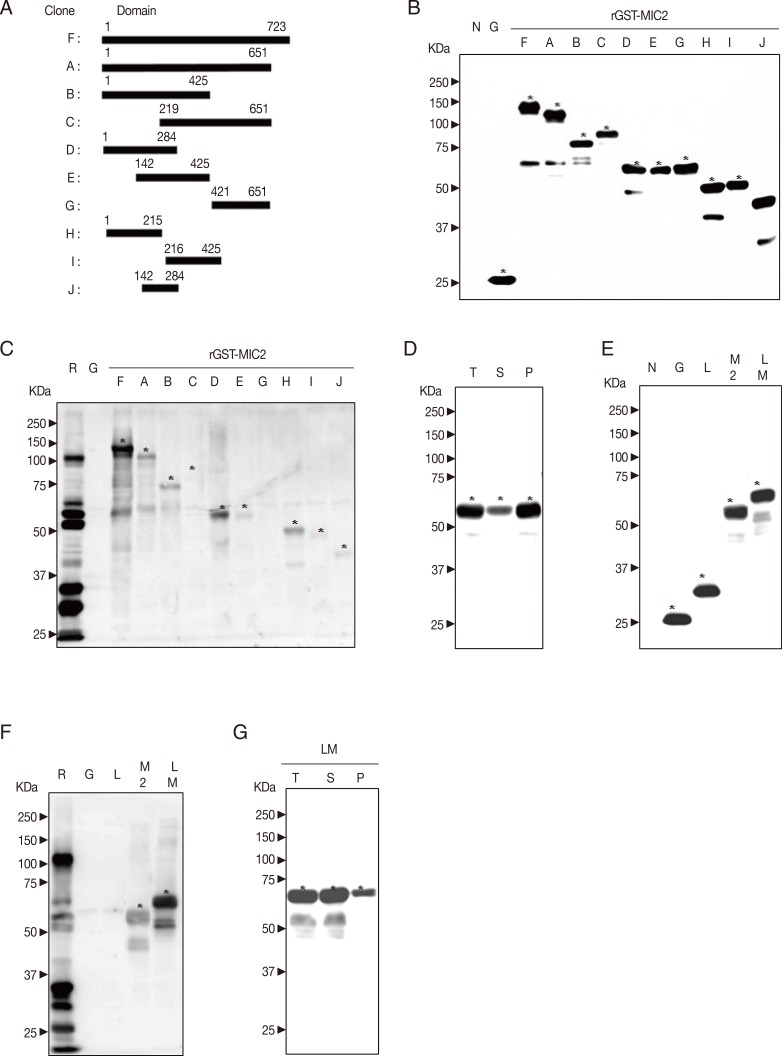
The main transmembrane region of MIC2 (GenBank no. XM_002367433.1) is predicted in its C-terminal, and the downstream half of the C-terminal sequence of MIC2 possesses higher disorder tendency. Based on the structure and results of bioinformatics analysis, 10 fragments of cDNA of MIC2 were subcloned into pGEX-4T-1 vector in
EcoRI or
XhoI sites. Cloning domains and corresponding sequences of cDNA are shown in
Fig. 4A.
All recombinant proteins in BL21 (DE3) pLysS
E. coli were induced at 30℃, 0.5 mM IPTG. As shown in
Fig. 4B, rGST-MIC2 fusion proteins were well expressed. In
Fig. 4C, rGST-MIC2
1-723 showed the strongest antigenicity and further dissected as rGST-MIC2
1-284 maintained strong antigenicity. Others possessed slight or no antigenicity. However, rGST-MIC2
1-284 is slightly soluble at 30℃, 0.5 mM IPTG in
Fig. 4D.
The pGEX-4T-1/GRA2
31-71 and PCR product of MIC2
1-284 were digested by
EcoRI and
XhoI restriction digestion enzymes. Two linear DNA were ligated and transformed into BL21 (DE3) pLysS
E. coli. In
Fig. 4E, rGST-GRA2
31-71-MIC2
1-284 protein was well expressed as much as rGST-MIC2
1-284. However, comparing to rGST-MIC2
1-284, rGST-GRA2
31-71-MIC2
1-284 protein was significantly increased the solubility at 30℃, 0.5 mM IPTG and possessed higher antigenicity in
Fig. 4F and 4G.
DISCUSSION
Currently, lots of recombinant antigens of
T. gondii have been developed and tested in ELISA and western blot for recognition of anti-
T. gondii antibodies [
5] because highly yielded recombinant antigen in prokaryotic expression system is not expensive and short-time consuming [
6]. However, the disadvantage of recombinant antigens is that the folding of recombinant antigens may be very different from those of native antigens [
7] to expose the antigenic epitope correctly. The solubility of recombinant antigens affects the application of them to prepare the serological test apparatus. To solve those problems, bioinformatics approaches were applied to predict the structure of proteins [
10,
22,
23,
24].
It is reported that a fly-casting model for intrinsically unstructured protein recognition [
25], which illustrates a relatively unstructured disordered protein molecule, is revealed to have a greater capture radius for a specific binding site with increasing flexibility [
26,
27]. The recombinant antigen fused with the IUD domain of GRA2 is significantly soluble and shows an enhanced antigenicity. In the present study, using bioinformatics tools, the protein structure of GRA2, GRA3, ROP2, and MIC2 genes were analyzed, which was revealed as major secretory/excretory antigens of
T. gondii as reported in our previous study [
21].
GRA2 is a promising candidate for serodiagnosis of toxoplasmaosis [
28]. Except the signal sequence, the whole domain of GRA2 is predicted as disordered region by bioinformatics tool. Seven fragments of GRA2 were cloned (
Fig. 1A), all of which were well expressed (
Fig. 1B). Recombinant proteins with 3 N-terminal domains of GRA2 showed respectively higher value of IgG avidity, comparing to other fusion proteins (
Fig. 1C). Among them, rGST-GRA2
25-105 is the shortest recombinant protein and has characteristics of water-soluble (
Fig. 1D) in this study. Therefore, GRA2
25-105 may be the main antigenic domain of GRA2 against patient serum.
GRA3 has 2 putative transmembrane regions and parasitophorous vacuolar membrane-associated GRA3 interacts with calcium modulating ligand of host cell endoplasmic reticulum [
29]. Two fragments of GRA3 were cloned (
Fig. 2A). Both of these 2 recombinant antigens showed the same antigenic ability against patient serum (
Fig. 2C). However, the recombinant protein fused with the domain of GRA3
39-222 was not well expressed and slightly soluble (
Fig. 2B, D) and furthermore the host BL21 (DE3) pLysS
E. coli containing the recombinant plasmid underwent degradation during the induction with IPTG. On the while, rGST-GRA3
39-138 was well expressed and significantly soluble as shown in
Fig. 2B and D. However, actual molecular weight of rGST-GRA3
39-138 estimated by SDS-PAGE analysis was not expected as calculated, which presumed due to the composition of amino acid residues [
30]. Thus, rGST-GRA3
39-138 may be a promising candidate to develop a serological diagnosis kit.
ROP2 is a kind of kinase, and the catalytic domain of it is located in C-terminal half [
16]. Three potential epitopes of ROP2 (197-216, 393-410, and 501-524) are recognized by human T cells [
31] and rROP2
196-561 has positive reactions to both Toxo-IgG and -IgM antibodies [
32]. Eight fragments of ROP2 were cloned (
Fig. 3A), and all fusion antigens were well expressed at indicated condition (
Fig. 3B). The rGST-ROP2
29-561 and rGST-ROP2
324-561 showed equivalent higher value of IgG avidity (
Fig. 3C) as paralleled with a previous study [
31], but rGST-ROP2
29-323 did not show antigenicity in the present study. Moreover, rGST-ROP2
324-561 was slightly soluble at the indicated conditions (
Fig. 3D) due to the well-folded structure of the catalytic domain of ROP2. The insolubility of rGST-ROP2
324-561 is a big obstacle to develop a serodiagnostic kit; therefore, the unfolded flexible linker was recommended in this study [
10]. The IUD region of GRA2
31-71 was subcloned into the recombinant antigen (
Fig. 3E), which resulted in the increase of solubility of the kimeric protein rGST-GRA2
31-71-ROP2
324-561 at the indicated condition comparing to rGST-ROP2
324-561 (
Fig. 3G). The antigenicity of rGST-GRA2
31-71-ROP2
324-561 was maintained as same as that of rGST-ROP2
324-561 (
Fig. 3F). The rGST-GRA2
31-71-ROP2
324-561 may be a promising candidate to develop a serological diagnosis kit.
MIC2 has a transmembrane region in its C-terminal region and has no signal peptides [
7]. Ten fragments of MIC2 were cloned (
Fig. 4A), and all fusion antigens were well expressed at the indicated condition (
Fig. 4B). The rGST-MIC2
1-723 and rGST-MIC2
1-284 showed higher values of IgG avidity comparing to other recombinant antigens (
Fig. 4C). One MIDAS and 1 TSP1 domains are involved in the amino acid 1-284 of MIC2 [
7]. However, rGST-MIC2
29-323 was slightly soluble at the indicated conditions (
Fig. 4D), which may be due to the well-folded structure of MIC2
1-284 domain. The rGST-MIC2
1-284 was not suitable to develop a serological diagnosis kit. The IUD region of GRA2
31-71 was subcloned into pGEX-4T-1/MIC2
1-284. As a result, rGST-GRA2
31-71-MIC2
1-284 was highly yielded and soluble (
Fig. 4E, G) at the indicated condition comparing to rGST-MIC2
1-284. Interestingly, comparing to rGST-MIC2
1-284, rGST-GRA2
31-71-MIC2
1-284 showed a higher value of IgG avidity (
Fig. 4F). It is strongly recommended that rGST-GRA2
31-71-MIC2
1-284 is a promising candidate to develop a serological diagnosis kit.
In summary, 4 promising recombinant antigens including 2 chimeric proteins were cloned. They have relatively lower molecular weight to enrich the number of antigens in a restricted condition of smaller diagnostic product with maintaining higher sensitivity. It is once again proven that IUD domain enhances the plasticity and solubility of proteins in the present study [
10,
26]. Therefore, based on the analysis of bioinformatics approaches, 4 recombinant antigens were cloned and promising candidates to develop serological diagnosis kits, for example ELISA or rapid diagnostic test (RDT).
NRF-2012R1A1A 2002612
Notes
-
We have no conflict of interest related to this study.
ACKNOWLEDGMENT
This research was partially supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology, Korea (NRF-2012R1A1A 2002612).
References
- 1. Tenter AM, Heckeroth AR, Weiss LM. Toxoplasma gondii: from animals to humans. Int J Parasitol 2000;30:1217-1258.
- 2. Wong SY, Remington JS. Toxoplasmosis in pregnancy. Clin Infect Dis 1994;18:853-861.
- 3. Choi WY, Nam HW, Kwak NH, Huh W, Kim YR, Kang MW, Cho SY. Foodborne outbreaks of human toxoplasmosis. J Infect Dis 1997;175:1280-1282.
- 4. Park YH, Han JH, Nam HW. Clinical features of ocular toxoplasmosis in Korean patients. Korean J Parasitol 2011;49:167-171.
- 5. Holec-Gasior L. Toxoplasma gondii recombinant antigens as tools for serodiagnosis of human toxoplasmosis: current status of studies. Clin Vaccine Immunol 2013;20:1343-1351.
- 6. Holec-Gasior L, Kur J. Toxoplasma gondii: recombinant GRA5 antigen for detection of immunoglobulin G antibodies using enzyme-linked immunosorbent assay. Exp Parasitol 2010;124:272-278.
- 7. Pietkiewicz H, Hiszczyńska-Sawicka E, Kur J, Petersen E, Nielsen HV, Paul M, Stankiewicz M, Myjak P. Usefulness of Toxoplasma gondii recombinant antigens (GRA1, GRA7 and SAG1) in an immunoglobulin G avidity test for the serodiagnosis of toxoplasmosis. Parasitol Res 2007;100:333-337.
- 8. Hiszczyńska-Sawicka E, Brillowska-Dabrowska A, Dabrowski S, Pietkiewicz H, Myjak P, Kur J. High yield expression and single-step purification of Toxoplasma gondii SAG1, GRA1, and GRA7 antigens in Escherichia coli. Protein Expr Purif 2003;27:150-157.
- 9. Huang X, Xuan X, Suzuki H, Sugimoto C, Nagasawa H, Fujisaki K, Mikami T, Igarashi I. Characterization of Toxoplasma gondii SAG2 expressed in insect cells by recombinant baculovirus and evaluation of its diagnostic potential in an enzyme-linked immunosorbent assay. Clin Diagn Lab Immunol 2002;9:1343-1347.
- 10. Song KJ, Yang Z, Chong CK, Kim JS, Lee KC, Kim TS, Nam HW. A rapid diagnostic test for toxoplasmosis using recombinant antigenic N-terminal Half of SAG1 linked with intrinsically unstructured domain of gra2 protein. Korean J Parasitol 2013;51:503-510.
- 11. Ching XT, Lau YL, Fong MY, Nissapatorn V. Evaluation of Toxoplasma gondii recombinant dense granular protein (GRA2) for serodiagnosis by western blot. Parasitol Res 2013;112:1229-1236.
- 12. Nam HW, Im KS, Baek EJ, Choi WY, Cho SY. Analysis of antigenic domain of GST fused major surface protein (p30) fragments of Toxoplasma gondii. Korean J Parasitol 1996;34:135-141.
- 13. Nam HW. GRA proteins of Toxoplasma gondii: maintenance of host-parasite interactions across the parasitophorous vacuolar membrane. Korean J Parasitol 2009;47(suppl):S29-S37.
- 14. Bermudes D, Dubremetz JF, Achbarou A, Joiner KA. Cloning of a cDNA encoding the dense granule protein GRA3 from Toxoplasma gondii. Mol Biochem Parasitol 1994;68:247-257.
- 15. Mercier C, Lecordier L, Darcy F, Deslee D, Murray A, Tourvieille B, Maes P, Capron A, Cesbron-Delauw MF. Molecular characterization of a dense granule antigen (Gra 2) associated with the network of the parasitophorous vacuole in Toxoplasma gondii. Mol Biochem Parasitol 1993;58:71-82.
- 16. Beckers CJ, Dubremetz JF, Mercereau-Puijalon O, Joiner KA. The Toxoplasma gondii rhoptry protein ROP 2 is inserted into the parasitophorous vacuole membrane, surrounding the intracellular parasite, and is exposed to the host cell cytoplasm. J Cell Biol 1994;127:947-961.
- 17. Carruthers VB, Tomley FM. Microneme proteins in apicomplexans. Subcell Biochem 2008;47:33-45.
- 18. Dosztányi Z, Csizmók V, Tompa P, Simon I. The pairwise energy content estimated from amino acid composition discriminates between folded and intrinsically unstructured proteins. J Mol Biol 2005;347:827-839.
- 19. Cserzö M, Wallin E, Simon I, von Heijne G, Elofsson GA. Prediction of transmembrane alpha-helices in prokaryotic membrane proteins: the dense alignment surface method. Protein Eng 1997;10:673-676.
- 20. Kim MH, Choi YK, Park YK, Nam HW. A toxoplasmic uveitis case of a 60-year-old male in Korea. Korean J Parasitol 2000;38:29-31.
- 21. Son ES, Nam HW. Detection and characterization of excretory/secretory proteins from Toxoplasma gondii by monoclonal antibodies. Korean J Parasitol 2001;39:49-56.
- 22. Bai Y, He S, Zhao G, Chen L, Shi N, Zhou H, Cong H, Zhao Q, Zhu XQ. Toxoplasma gondii: bioinformatics analysis, cloning and expression of a novel protein TgIMP1. Exp Parasitol 2012;132:458-464.
- 23. Macêdo AG Jr, Cunha JP Jr, Cardoso TH, Silva MV, Santiago FM, Silva JS, Pirovani CP, Silva DA, Mineo JR, Mineo TW. SAG2A protein from Toxoplasma gondii interacts with both innate and adaptive immune compartments of infected hosts. Parasit Vectors 2013;6:163.
- 24. Zhao G, Zhou A, Lu G, Meng M, Sun M, Bai Y, Han Y, Wang L, Zhou H, Cong H, Zhao Q, Zhu XQ, He S. Identification and characterization of Toxoplasma gondii aspartic protease 1 as a novel vaccine candidate against toxoplasmosis. Parasit Vectors 2013;6:175.
- 25. Shoemaker BA, Portman JJ, Wolynes PG. Speeding molecular recognition by using the folding funnel: the fly-casting mechanism. Proc Natl Acad Sci USA 2000;97:8868-8873.
- 26. Tompa P. The interplay between structure and function in intrinsically unstructured proteins. FEBS Lett 2005;579:3346-3354.
- 27. Uversky VN, Dunker AK. The case for intrinsically disordered proteins playing contributory roles in molecular recognition without a stable 3D structure. F1000 Biol Rep 2013;5:1.
- 28. Zhou H, Gu Q, Zhao Q, Zhang J, Cong H, Li Y, He S. Toxoplasma gondii: expression and characterization of a recombinant protein containing SAG1 and GRA2 in Pichia pastoris. Parasitol Res 2007;100:829-835.
- 29. Kim JY, Ahn HJ, Ryu KJ, Nam HW. Interaction between parasitophorous vacuolar membrane-associated GRA3 and calcium modulating ligand of host cell endoplasmic reticulum in the parasitism of Toxoplasma gondii. Korean J Parasitol 2008;46:209-216.
- 30. Mercier C, Adjogble KD, Däubener W, Delauw MF. Dense granules: are they key organelles to help understand the parasitophorous vacuole of all apicomplexa parasites? Int J Parasitol 2005;35:829-849.
- 31. Saavedra R, Becerril MA, Dubeaux C, Lippens R, De Vos MJ, Hérion P, Bollen A. Epitopes recognized by human T lymphocytes in the ROP2 protein antigen of Toxoplasma gondii. Infect Immun 1996;64:3858-3862.
- 32. Martin V, Arcavi M, Santillan G, Amendoeira MRR, De Souza NE, Griemberg G, Guarnera E, Garberi JC, Angel SO. Detection of human Toxoplasma-specific immunoglobulins A, M, and G with a recombinant Toxoplasma gondii Rop2 protein. Clin Diagn Lab Immunol 1998;5:627-631.
Fig. 1Production of GST-GRA2 fusion protein. "N" indicates lysate of BL21 (DE3) pLysS E. coli without induction; "G", lysate of E. coli transformed with vector after induction; "R", Toxoplasma lysate antigen (TLA) of RH strain; "T", total lysates of E. coli; "S", soluble fraction; and "P", insoluble fraction. The meaning of abbreviations is the same in the following context without extra illustration. (A) Design of fragmentation of cDNA of GRA2; 7 fragments of GRA2 were cloned. The name of clones and amino acid regions are indicated. "F", full sequence of GRA2 without signal sequence; "A", 2/3 N-terminal (Nt); "B", 2/3 C-terminal (Ct); "C", half Nt; "D", half Ct; "E", middle 1/3 sequence; and "L", linker, is the high disorder sequence of Nt. (B) Expression of recombinant GRA2 antigens induced at 30℃, 0.5 mM IPTG. Target bands against GST by western blot were marked with asterisks. (C) Antigenicity of recombinant GRA2 antigens. Patient serum was applied to detect the antigenicity of recombinant proteins against human IgG by western blot. The detectable signal was marked with asterisks. (D) Solubility of recombinant GRA2 antigens. The solubility of rGST-GRA225-105 was tested by western blot against GST.

Fig. 2Production of GST-GRA3 fusion protein. (A) Design of fragmentation of cDNA of GRA3. Two fragments of GRA3 were cloned. "F", full sequence of GRA3 without signal sequence and "A", half Nt GRA3 without predicted Nt and Ct transmembrane domains. (B) Expression of recombinant GRA3 antigens. rGST-GRA339-138 was well expressed, but rGST-GRA339-222 was not. Target bands against GST by western blot were marked with asterisks. (C) Antigenicity of recombinant GRA3 antigens. The results of western blot against patient serum are shown here as the 2 clones show the same antigenicity. Target bands were indicated with asterisks. (D) Solubility of recombinant GRA3 antigens. The NC membrane was incubated with rabbit anti-GST antibody.

Fig. 3Production of GST-ROP2 fusion protein. (A) Design of fragmentation of cDNA of ROP2. Eight fragments of ROP2 were cloned as "A", full sequence of ROP2 without signal sequence; "B", 1/2 Nt sequence of ROP2; "C", 1/2 Ct containing kinase domain; "D", 1/4 Nt; "E", middle 1/4 Ct; "G", 1/4Ct; "H", 1/2 Nt of kinase domain; and "I", 1/2 Ct of kinase domain. (B) Expression of recombinant ROP2 antigens. All recombinant proteins were well induced and tested by western blot with anti-GST antibody. (C) Antigenicity of recombinant ROP2 antigens. The antigenicity was tested by western blot against patient serum. (D) Solubility of recombinant ROP2 antigens. Solubility of rGST-ROP2324-561 induced was confirmed by western blot with anti-GST antibody. (E) Expression of recombinant linker ROP2 antigens. "L", lysate of BL21 (DE3) pLysS E. coli transformed with pGEX-4T-1/GRA231-71; "R2", that with pGEX-4T-1/ROP2324-561; and "LR", that with pGEX-4T-1/GRA231-71-ROP2324-561. The expression was confirmed by western blot with anti-GST antibody. (F) Antigenicity of recombinant linker ROP2 antigens. It was tested by western blot against patient serum. (G) Solubility of recombinant linker ROP2 antigens. Soluble and insoluble fractions of rGST-GRA231-71-ROP2324-561 protein were tested by western blot.

Fig. 4Production of GST-MIC2 fusion protein. (A) Design of fragmentation of cDNA of MIC2. Ten fragments of MIC2 were cloned as "F", full sequence of MIC2; "A", full sequence without Ct transmembrane region; "B", 2/3 Nt; "C", 2/3 Ct; "D", 1/3 Nt; "E", middle 1/3; "G", 1/3 Ct; "H", half Nt of D; "I", half Ct of D; and "J", middle 1/3 of D. (B) Expression of recombinant MIC2 antigens. All recombinant proteins were well induced and tested by western blot against anti-GST antibody. (C) Antigenicity of recombinant MIC2 antigens. The antigenicity of GST-MIC2 was tested by western blot against patient serum. (D) Solubility of recombinant MIC2 antigens. Total lysate, soluble, and insoluble fractions of rGST-MIC21-284 were detected against GST antibody. (E) Expression of recombinant linker MIC2 antigens. BL21 (DE3) pLysS E. coli containing GST and GST linker MIC21-284 recombinant plasmids were induced; "L", lysate transformed with pGEX-4T-1/GRA231-71; "M2", that with pGEX-4T-1/MIC21-284; and "LM", that with pGEX-4T-1/GRA231-71-MIC21-284. The expression was confirmed against anti-GST antibody. (F) Antigenicity of recombinant linker MIC2 antigens. It was tested against patient serum. (G) Solubility of recombinant linker MIC2 antigens. The soluble and insoluble fractions of rGST-GRA231-71-MIC21-284 protein were tested by western blot.

Table 1.Primers designed for the amplification of T. gondii genes fragments
Table 1.
|
Gene fragments |
|
Sequence of primers |
|
|
GRA2 |
F, A, C |
Sense |
5´-CCG GAATTC GAGTTTTCCGGAGTTGTTAACC-3´ |
|
F, B, D |
Antisense |
5´-CCG CTCGAG CTGCGAAAAGTCTGG-3´ |
|
A, E |
Antisense |
5´-CCG CTCGAG CACCATGCCCCTTCC-3´ |
|
B, E |
Sense |
5´-CGG GAATTC GCATCCAGAGTGGCAGAAC-3´ |
|
C |
Antisense |
5´-CCG CTCGAG CTTTGCTTTTTTGAAGGC-3´ |
|
D |
Sense |
5´-CG GAATTC GTGGTGGCAGAAAAAGGC-3´ |
|
L |
Sense |
5´-CG GGATCC CAGGGACCAGTCGAC-3´ |
|
L |
Antisense |
5´-CG GGATCC AACCGGTTCTTCTGGCT-3´ |
|
GRA3 |
F, A |
Sense |
5´-G GAATTC GGCCTTGCGGCGGAT-3´ |
|
F |
Antisense |
5´-CCG CTCGAG AGCACGCTTCAA ACC A-3´ |
|
A |
Antisense |
5´-CCG CTCGAG GGTTTGTTTCTTGGAGG-3´ |
|
ROP2 |
A, B, D |
Sense |
5´-CG GAATTC CAAGGCGCTGGCGTT-3´ |
|
A, C, G, I |
Antisense |
5´-CG GAATTC TGCCGGTTCTCCATCAGT-3´ |
|
B |
Antisense |
5´-CGG GAATTC AAATCTGAGATACGCCTTGGC-3´ |
|
C, E, H |
Sense |
5´-CCGG GAATTC ATATTCCCCATCGATTTGGTG-3´ |
|
D |
Antisense |
5´-CG GAATTC AGGATCCGTACCGCG-3´ |
|
E |
Antisense |
5´-CCG CTCGAG AATCCAGTAGAT-3´ |
|
G |
Sense |
5´-CGG GAATTC TATGGCCTTGTGCATGC-3´ |
|
H |
Antisense |
5´-CCG CTCGAG ATGTTCAAAGCCGGT-3´ |
|
I |
Sense |
5´-CG GAATTC CTGGTGCGAGACGG-3´ |
|
MIC2 |
F, A, B, D, H |
Sense |
5´-CCG GAATTC ATGTGTGTGCTCGTTCCT-3´ |
|
F |
Antisense |
5´-CCG CTCGAG CTCCATCCACATATCACTATC-3´ |
|
A, C, G |
Antisense |
5´-CCG CTCGAG ACTGCCTGACTCTTTCT-3´ |
|
B, E, I |
Antisense |
5´-CCG CTCGAG TGCATTAATTGGACACG-3´ |
|
C |
Sense |
5´-CCG GAATTCACACTCCCCCAGGAT-3´ |
|
D, J |
Antisense |
5´-CCG CTCGAG TTCACGAATTTCTTCAAGTCC-3´ |
|
E, J |
Sense |
5´-CCG GAATTC GATGGCGAATCGGATTCT-3´ |
|
G |
Sense |
5´-CGG GAATTC ACTTGCGGTCAGTTTGAAGA-3´ |
|
H |
Antisense |
5´-CCG CTCGAG TTTAAGCATCGGTTTAATCGC-3´ |
|
I |
Sense |
5´-CCG GAATTC GAGGTTTGTAAGACACTCCC-3´ |