Abstract
The determination of the accurate immune status of pregnant women is crucial in order to prevent congenital toxoplasmosis. Equivocal results with conventional serological techniques are not uncommon when IgG titers are close to the cut-off value of the test, so that a confirmatory technique is needed. For this purpose, we developed a homemade immunoblot (IB) using soluble extract of Toxoplasma gondii tachyzoites and assessed it by testing 154 positive, 100 negative, and 123 equivocal sera obtained from pregnant women. In order to select the more valuable bands in terms of sensitivity and specificity, we used the Youden Index (YI). The highest YIs were those given by the 32, 36, 98, 21, and 33 bands. The simultaneous presence on the same blot of at least 3 bands showed a much higher YI (0.964) and was adapted as the positivity criterion. The analysis of results showed that our homemade IB correlated well with the commercial LDBIO Toxo II IgG® kit recently recommended as a confirmatory test (96.7% of concordance).
-
Key words: Toxoplasma gondii, toxoplasmosis, serodiagnosis, immunoblot, soluble tachyzoite antigen
INTRODUCTION
Toxoplasmosis is the most frequent and widespread protozoal infection in humans. Although usually asymptomatic or benign in immunocompetent individuals, toxoplasmosis may cause severe lesions in the fetus when acquired by non-immune pregnant women [
1,
2]. As congenital transmission can only occur in previously seronegative women, the determination and correct interpretation of the immune status are essential for an appropriate prophylactic measures and treatment [
3,
4]. In immunocompetent subjects, the diagnosis of toxoplasmosis is commonly established by the detection of specific anti-
Toxoplasma IgG and IgM antibodies in the serum. Despite the availability of a great variety of serological tests and efforts for international standardization, discordance between techniques and equivocal results are not uncommon when the amounts of specific IgG are too low or close to the cut-off value of the test (gray zone) [
5,
6]. In our laboratory, where enzyme immunoassay (EIA-IgG) and fluorescent antibody test (FAT-IgG) are used in routine, borderline results account for nearly 4% of tested sera [
5]. The difficulties in interpretation of borderline results encountered when the quantitative enzyme immunosystems are used and the discrepancies between tests are, for a great part, caused by the differences between the antigenic preparations, and the respective proportions of soluble or membrane antigens [
7,
8,
9]. In these situations, the precise determination of the immune status may be problematic, so that a confirmatory test is highly needed [
5,
6,
10]. This is obviously of a major concern in pregnant women because of the risk of congenital toxoplasmosis. Moreover, as soon as the immune status is unequivocally determined, no further testing is needed for seropositive women who are considered to be im-munized. In contrast, a monthly serological monitoring is essential for non-immune patients.
The dye test of Sabin and Feldman has long been the gold standard method in the serodiagnosis of toxoplasmosis owing to its high specificity and sensitivity, but it is labor-in-tensive, tedious, not commercially available, and neither suitable for routine use, so that it is only used by a very few laboratories [
2,
6]. The LDBio-Toxo II IgG Western blot confirmation® test (LDBIO Diagnostics, Lyon, France) (LDBIO II), which detects specific anti-
T. gondii IgG, was recently developed to confirm results of conventional quantitative tests mainly when the IgG titers are very close to the cut-off value. The LDBIO II was found to be highly correlated to the dye test in terms of sensitivity and specificity [
2,
6,
10]. Its major drawback resides in its high cost which limits its use for routine analysis.
The aim of our work was to develop an immunoblot (IB) test that can be used as a confirmatory technique for discordant or equivocal results in enzyme immune assay (EIA) and indirect fluorescent antibody test (FAT).
MATERIALS AND METHODS
Sera
We tested 377 sera collected from pregnant women for a routine toxoplasmosis screening. The sera were divided into 3 groups:
Group I: comprised 154 positive sera in routine tests (EIA-IgG and FAT-IgG) with a titer ranging from 12 IU/ml to >240 IU/ml in EIA-IgG and from 12 IU/ml to 1736 IU/ml in FAT-IgG. Among them, 37 were positive in EIA-IgM.
Group II: comprised 100 sera with negative results in the same routine tests. Group I and Group II were designed for identifying the relevant antigenic bands in our homemade IB test.
Group III: comprised 123 sera with equivocal or discordant results in EIA-IgG and FAT. Sera with IgG titers ranging in the "gray zone" in EIA-IgG, FAT, or both were considered equivocal. Sera were considered discordant in the following situations: (i) serum positive in one technique and negative in the second, (ii) serum equivocal in one technique and negative or positive in the second one. The Group III was designed to assess our homemade IB test in detecting T. gondii-IgG antibodies in sera with doubtful results in routine tests.
EIA tests
EIA-IgG and EIA-IgM were performed manually by using the Platelia-Toxo IgG® and the Platelia-Toxo IgM® immunoassay (BioRad, Marnes-La-Coquette, France), according to the manufacturers' guide. In EIA-IgG, the results were expressed as international units per milliliter (IU/ml) and their interpretation was based on manufactures' criteria. The test was regarded positive if ≥9.0, negative if <6.0 and equivocal if ≥6.0 and <9.0 (gray zone). In EIA-IgM, results were expressed as positive, negative, or borderline according to the manufacturer's recommendations. The test was considered negative if the ratio was <0.8, equivocal if ≥0.8 and <1.0 (gray zone), and positive if ≥1.0.
FAT
This test was carried out using the ToxoSpot IF® commercially available slides (BioMérieux, Marcy l'Etoile, France). The titers of sera were calculated by reference to a positive control with known titer and expressed as IU/ml. The test was positive if ≥12.0, negative if <6.0, and equivocal if ≥6.0 and <12.0 (gray zone).
LDBIO-Toxo II IgG
We used the LDBIO-Toxo II IgG western Blot® (LDBIO, Lyon, France) according to the manufacturer's guide. The resulting bands on the patient's serum strip were compared with the 5 following bands on the positive control strip: 30, 31, 33, 40, and 45 kDa. A positive result is defined by the presence of at least 3 matching bands on the patient's strip, including the 30 kDa band. For Group III, the LDBIO II was used as a confirmatory
technique for sera with equivocal results.
Homemade immunoblot with soluble tachyzoite antigens
Soluble tachyzoite antigens was prepared as previously described [
11,
12]. Briefly, female Swiss albino mice, weighing 30-35 g were intraperitoneally injected with a 10
3-10
4/ml suspension of the virulent RH
T. gondii strain tachyzoites. Parasites obtained from the peritoneal exudate of mice were washed 3 times with PBS (pH 7.2-7.4) by centrifugation at 1,500
g for 15 min and adjusted to10
8 tachyzoites/ml. The parasite pellets were subjected to 5-thaw cycles using liquid nitrogen and 37℃ water bath. Tachyzoites were further disrupted by sonication in ice using a sonicator (80W, 20 sec duration, 20 times, with 10 sec intervals). The lysate was centrifuged at 17,500
g for 30 min at 4℃. The protein concentration of the resulting supernatant, containing soluble antigens, was measured by the Bradford method using BSA as a standard.
SDS-PAGE was performed according to the method of Laemmli [
13]. Proteins and molecular weight (MW) marker (See Blue® Pre-stained Standard, Invitrogen, Carlsbad, California, USA) were separated on 12% acrylamide gel (Mini-Protean Tetra Cell, BioRad, Hercules, California, USA) under reducing conditions. After migration, the gel was stained with Coomassie Blue G250 (BioRad). The images of the gel were analyzed using the ChemiDoc™ MP (BioRad) and Quantity One Software package for imaging and analyzing 1-D electrophoresis gels.
For IB, the separated proteins were transferred from the gel to a nitrocellulose membrane (90V, 350 mA for 90 min). The membrane was blocked in 5% dried skimmed milk in PBS-0.3% Tween 20 for 2 hr at room temperature, then incubated overnight at 4℃ with the diluted patients' sera (1/100). After 5 washes for 5 min in PBS-T and incubation with alkaline phosphatase-goat anti-human IgG antibody (1/6,000) for 90 min, the NBT/BCIP substrate (Invitrogen) was used for the revelation of bands according to the manufacturer's instructions. The revelation was blocked with 2 washes of ddH2O. The MW of the revealed bands was determinate by using the ChemiDoc™ MP and Quantity One software package (BioRad).
Statistical analysis
The χ
2-test was used to compare the frequencies of each band between positive and negative sera. For the comparison of the sensitivity and specificity of the bands, we used the χ
2-test of Mc Nemar. To evaluate the diagnostic value of each band, we used the Youden index (IY) which takes into account the specificity and sensitivity of the diagnostic test [
14]. This index is calculated according to the following formula: IY=(sensitivity + specificity)-1. It ranges between -1 and 1. The test giving the highest index (the closer to 1) is considered to have the best diagnostic performance.
RESULTS
Homemade IB with sera of Group I and Group II
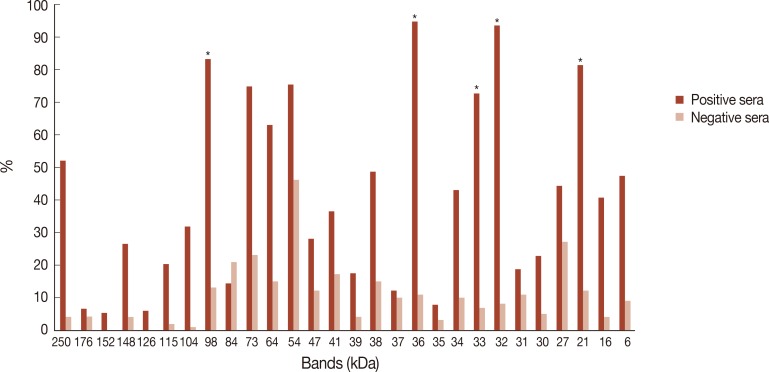
A total of 28 bands with a MW ranging from 6 to 250 kDa were detected by both positive and negative sera. These bands were the followings: 250, 176, 152, 148, 126, 115, 104, 98, 84, 73, 64, 54, 47, 41, 39, 38, 37, 36, 35, 34, 33, 32, 31, 30, 27, 21, 16, and 6 kDa, except for the 152 kDa and 126 kDa bands that were only observed with 8 and 1 positive sera, respectively (
Fig. 1).
The number of bands per positive serum ranged from 5 to 20 bands with a mean of 11.6 bands per serum. The number of bands per negative serum ranged from 0 to 12 with a mean of 3.0 bands per serum.
Out of the 28 bands, the 32, 36, 98, 21, and 33 kDa showed the highest YIs (0.855, 0.838, 0.701, 0.692, and 0.657, respectively) and were selected as the most relevant markers of infection (
Table 1).
As none of the 5 selected bands showed neither an absolute specificity nor an absolute sensitivity, we calculated the YI given by the association in the same blot of 2 or more out of the 5 bands. The highest YI (0.964) was that shown by the simultaneous presence of at least 3 bands which we considered as a criterion of positivity (
Table 2).
According to these finding, we propose the following 2 interpretations: 1) The presence on the same blot of at least 3 of the 32, 36, 98, 21, and 33 kDa attests the presence of anti-Toxoplasma IgG antibodies in the tested serum. 2) The absence of the 5 bands or the presence of 2 or 1 single band attests the absence of anti-Toxoplasma IgG antibodies in the tested serum.
Homemade IB test with Group III
Among the 123 sera with discordant or equivocal results, 107 were positive and 16 were negative in LDBIO II test. Among the 107 positive sera in LDBIO II, 103 (97.14%) were positive in our homemade IB test. The 16 negative sera in LDBIO Toxo II IgG were all negative in the homemade IB test. The concordance between LDBIO II test and our IB test was 96.7%.
Figs. 2,
3 illustrate some profiles obtained with positive and negative sera in LDBIO II and in our homemade IB, respectively.
DISCUSSION
The determination of the accurate immune status of pregnant women mainly with regard to the IgG response is essential for evaluating and preventing the risk of congenital toxoplasmosis. Most of the commercially available tests for IgG detection are overall reliable and satisfactory in terms of sensitivity and specificity [
5,
6]. However, laboratories are often faced with interpretation problems when using conventional methods mainly when IgG concentrations are too low or very close to the cut-off value of the technique, which leads to equivocal results [
5,
10]. In these situations, it is usually recommended to test the same serum in a second technique, even though in most cases, the immune status of the patient remains doubtful. This highlights the need for a confirmatory test to check samples with borderline results in order to unambiguously determine the correct immune status of the investigated patients.
The LDBIO II was reported to be very valuable as a confirmatory test and shown to be highly correlated to the dye test considered so far as the gold standard [
15]. However, the test is not suitable for a routine use because of its high cost and because no information is available about the preparation of antigens and confection of strips. For these reasons, we developed a homemade IB test using soluble antigens extracted from tachyzoites of
T. gondii, and assessed it towards equivocal sera obtained from pregnant women. The analysis of the IB patterns showed that, as expected, the positive sera were more reactive than the negative sera with respect to the number of bands per serum (11.63 vs 2.98).
In order to select the more valuable bands as potential diagnostic markers, we used the YI which takes into account both the sensitivity and the specificity parameters. The highest YIs were given by the 5 following bands: 32, 36, 98, 21, and 33 kDa. The 32 kDa band, which presumably corresponds to the GRA6 protein, showed the highest YI (0.855) [
16]. The 32 kDa band is considered as a major
T. gondii antigen [
17,
18]. In addition it was reported to be a good marker of acute toxoplasmosis when tested in avidity-IB [
19].
The 36 kDa band showed the second highest YI (0.838). This band very probably corresponds to the Rop 9 protein and has been reported as a good serological marker of toxoplasmosis [
20,
21,
22]. The 98 kDa band showed a YI of 0.701 (the third highest YI). Even though the bands of high MW are often considered as poorly specific [
21], we could not discard the 98 kDa band as a potential serological marker because its sensitivity and specificity were quite satisfactory (83.1% and 87.0%, respectively). A band with the same MW has already been described by Johnson et al. [
23] in
T. gondii soluble extract. This band may be similar to the 97 kDa band described by Marcolino et al. [
19] and shown to be a good marker of recent disease in IB-avidity assay. In our study, the 98 kDa band was revealed by 128 positive sera regardless of the stage of the disease.
The 21 kDa and the 33 kDa bands gave the lowest YIs (0.692 and 0.657, respectively). Both antigens have already been reported by Fatoohi et al. [
21]. It is worth to note that the 33 kDa band is one of the 5 bands selected by the LDBIO II [
6]. In a previous report, the 21 kDa band showed a sensitivity of 71% when used as antigen in an EIA assay [
24]. On the other hand, the likeliness of the identify between the 21 kDa band and the 22 kDa bands described as a very interesting markers of toxoplasmosis cannot be discarded [
18,
25].
The bands identified as potential diagnostic markers with our test (21, 32, 33, 36, and 98 kDa) are different from those proposed by the LDBIO II kit (30, 31, 33, 40, and 45 kDa). This could be explained by technical differences in various parameters of the immunoblot such as the type of the antigen used (soluble, membranal, or total lysate) and its preparation, the SDS-PAGE and the immunoblotting itself. These parameters are not specified by the supplier of LDBIO II kit; therefore, we cannot compare them with the parameters of our test.
The analysis of our results showed that none of the revealed bands had an absolute sensitivity and specificity. In order to improve the performance of our homemade IB, we assessed the combination on the same blot of 2 or more of the 5 relevant bands. The highest YI was that generated by the association of at least 3 bands which we considered as a criterion of positivity in the interpretation of our IB patterns. When this criterion was applied to the 123 sera that showed equivocal results in conventional techniques (EIA and FAT), the sensitivity and the specificity of our IB were 97.1% and 100%, respectively. The concordance between our homemade IB and LDBIO II test was very high (96.7%).
The 4 false negative results shown by our homemade IB are none of major concern because the 4 women will be considered not immunized and further improperly followed up and submitted to the recommended preventive measures. In contrast, a false positive result would expose the misdiagnosed women to the risk of pergravidic toxoplasmic infection as they will be considered as immunized.
Notes
-
The authors declared no conflicts of interest with respect to the research, authorship, and/or publication of this article.
ACKNOWLEDGMENTS
Special thanks to Mr. Iheb Bougmiza, Associate Professor in the Faculty of Medicine of Sousse, Tunisia, for his contribution in the statistical analysis, and Mrs. Olfa Souissi, Superior Technician in Biology in the Laboratory of Parasitology of Pasteur Institute, Tunis for maintaining the RH Toxoplasma gondii strain and the manipulation of mice.
Ethical statement: The authors declare that the experiments comply with the current laws of the country in which they were performed (Tunisia).
References
- 1. Dunn D, Wallon M, Peyron F, Petersen E, Peckham C, Gilbert R. Mother-to-child transmission of toxoplasmosis: risk estimates for clinical counselling. Lancet 1999;353:1829-1833.
- 2. Flori P, Chene G, Varlet MN, Sung RT. Toxoplasma gondii serology in pregnant woman: characteristics and pitfalls. Ann Biol Clin (Paris) 2009;67:125-133.
- 3. Meroni V, Genco F. Toxoplasmosis in pregnancy: evaluation of diagnostic methods. Parassitologia 2008;50:51-53.
- 4. Sensini A. Toxoplasma gondii infection in pregnancy: opportunities and pitfalls of serological diagnosis. Clin Microbiol Infect 2006;12:504-512.
- 5. Khammari I, Saghrouni F, Yaacoub A, Gaied Meksi S, Ach H, Garma L, Fathallah A, Ben Saïd M. IgG western blot for confirmatory diagnosis of equivocal cases of toxoplasmosis by EIA-IgG and fluorescent antibody test. Korean J Parasitol 2013;51:485-488.
- 6. Franck J, Garin YJ, Dumon H. LDBio-Toxo II immunoglobulin G Western blot confirmatory test for anti-toxoplasma antibody detection. J Clin Microbiol 2008;46:2334-2338.
- 7. Cimon B, Marty P, Morin O, Bessières MH, Marx-Chemla C, Gay-Andrieu F, Thulliez P. Specificity of low anti-Toxoplasma IgG titers with IMx and AxSYM Toxo IgG assays. Diagn Microbiol Infect Dis 1998;32:65-67.
- 8. Derouin F, Garin YJ, Buffard C, Berthelot F, Petithory JC. Multicenter study of toxoplasma serology using various commercial ELISA reagents. Work Group Toxoplasmosis of the National Agency for Quality Control in Parasitology. Bull World Health Organ 1994;72:249-256.
- 9. Petithory JC, Ambroise-Thomas P, De Loye J, Pelloux H, Goullier-Fleuret A, Milgram M, Buffard C, Garin JP. Serodiagnosis of toxoplasmosis: a comparative multicenter study of a standard scale through various actual tests and expression of the results in international units. Bull World Health Organ 1996;74:291-298.
- 10. Leslé F, Touafek F, Fekkar A, Mazier D, Paris L. Discrepancies between a new highly sensitive Toxoplasma gondii ELISA assay and other reagents: interest of Toxo IgG western blot. Eur J Clin Microbiol Infect Dis 2011;30:1207-1212.
- 11. Kahi S, Cozon GJ, Greenland T, Wallon M, Gay-Andrieu F, Peyron F. A rapid flow cytometric method to explore cellular immunity against Toxoplasma gondii in humans. Clin Diagn Lab Immunol 1998;5:745-748.
- 12. Ma GY, Zhang JZ, Yin GR, Zhang JH, Meng XL, Zhao F. Toxoplasma gondii: proteomic analysis of antigenicity of soluble tachyzoite antigen. Exp Parasitol 2009;122:41-46.
- 13. Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970;227:680-685.
- 14. Perkins NJ, Schisterman EF. The Youden Index and the optimal cut-point corrected for measurement error. Biom J 2005;47:428-441.
- 15. Reiter-Owona I, Petersen E, Joynson D, Aspöck H, Dardé ML, Disko R, Dreazen O, Dumon H, Grillo R, Gross U, Hayde M, Holliman R, Ho-Yen DO, Janitschke K, Jenum PA, Naser K, Olszewski M, Thulliez P, Seitz HM. The past and present role of the Sabin-Feldman dye test in the serodiagnosis of toxoplasmosis. Bull World Health Organ 1999;77:929-935.
- 16. Gendrin C, Bittame A, Mercier C, Cesbron-Delauw MF. Post-translational membrane sorting of the Toxoplasma gondii GRA6 protein into the parasite-containing vacuole is driven by its N-terminal domain. Int J Parasitol 2010;40:1325-1334.
- 17. Lövgren K, Uggla A, Morein B. A new approach to the preparation of a Toxoplasma gondii membrane antigen for use in ELISA. Zentralbl Veterinarmed B 1987;34:274-282.
- 18. Sharma SD, Mullenax J, Araujo FG, Erlich HA, Remington JS. Western blot analysis of the antigens of Toxoplasma gondii recognized by human IgM and IgG antibodies. J Immunol 1983;131:977-983.
- 19. Marcolino PT, Silva DA, Leser PG, Camargo ME, Mineo JR. Molecular markers in acute and chronic phases of human toxoplasmosis: determination of immunoglobulin G avidity by western blotting. Clin Diagn Lab Immunol 2000;7:384-389.
- 20. Reichmann G, Dlugonska H, Fischer HG. Characterization of TgROP9 (p36), a novel rhoptry protein of Toxoplasma gondii tachyzoites identified by T cell clone. Mol Biochem Parasitol 2002;119:43-54.
- 21. Fatoohi AF, Cozon GJ, Gonzalo P, Mayencon M, Greenland T, Picot S, Peyron F. Heterogeneity in cellular and humoral immune responses against Toxoplasma gondii antigen in humans. Clin Exp Immunol 2004;136:535-541.
- 22. Attallah AM, Ismail H, Ibrahim AS, Al-Zawawy LA, El-Ebiary MT, El-Waseef AM. Immunochemical identification and detection of a 36-kDa Toxoplasma gondii circulating antigen in sera of infected women for laboratory diagnosis of toxoplasmosis. J Immunoassay Immunochem 2006;27:45-60.
- 23. Johnson AM, McDonald PJ, Neoh SH. Molecular weight analysis of soluble antigens from Toxoplasma gondii. J Parasitol 1983;69:459-464.
- 24. Holec-Gasior L, Kur J. Toxoplasma gondii: recombinant GRA5 antigen for detection of immunoglobulin G antibodies using enzyme-linked immunosorbent assay. Exp Parasitol 2010;124:272-278.
- 25. Ali-Heydari S, Keshavarz H, Shojaee S, Mohebali M. Diagnosis of antigenic markers of acute toxoplasmosis by IgG avidity immunoblotting. Parasite 2013;20:18.
Fig. 1Frequencies of antigenic bands recognized by IgG antibodies in positive (Group I) and negative sera (Group II). *Antigenic bands with a relevant Youden Index are indicated with an asterisk.

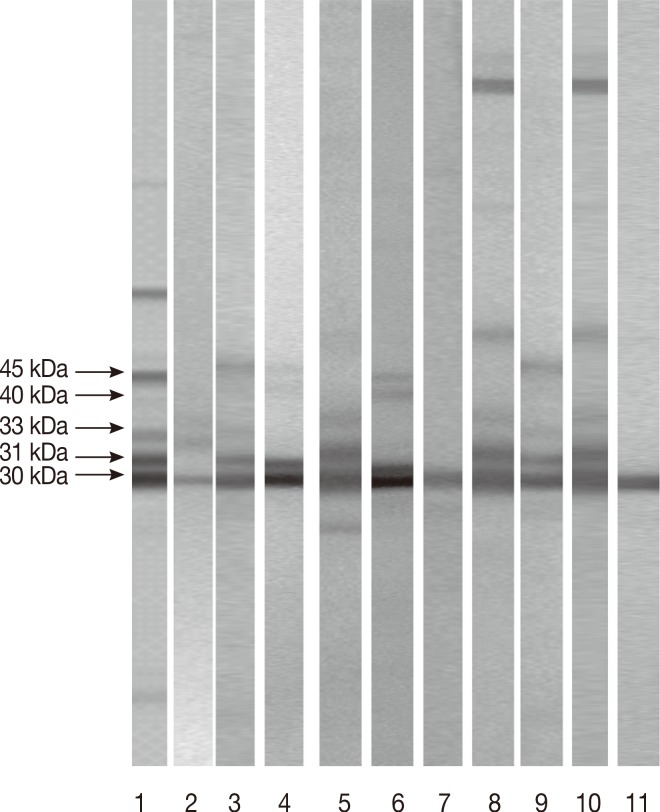
Fig. 2Patterns shown by equivocal and/or discordant sera (Group III) tested in LDBIO Toxo II IgG®. Lane 1: positive control with the 45, 40, 33, 31, and 30 kDa. Lane 2: negative sera with the 30 kDa. Lane 3: positive sera with the 30, 31, and 33 kDa. Lane 4: negative sera with the 30 and 31kDa. Lane 5: negative sera with the 30 and 31 kDa. Lane 6: positive sera with the 30, 31, 40, and 45 kDa. Lane 7: sera with the 30 kDa. Lane 8: positive sera with the 30, 31, and 33 kDa. Lane 9: positive sera with the 30, 31, and 33 kDa. Lane 10: negative sera with the 30 and 31 kDa. Lane 11: negative sera with the 30 kDa.

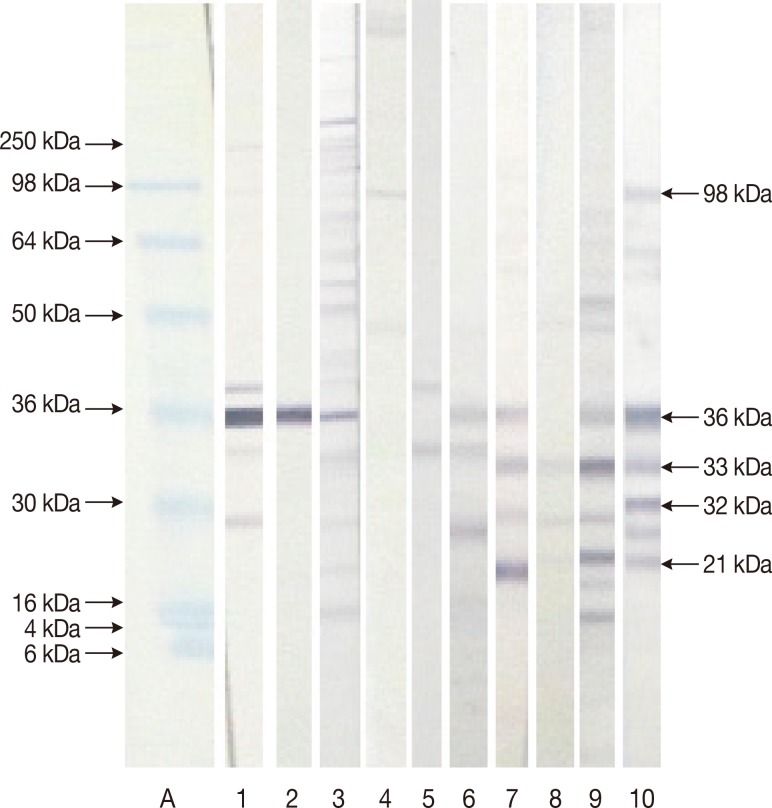
Fig. 3Patterns shown by equivocal and discordant sera (Group III) tested in our homemade immunoblot. Lane A: molecular weight marker. Lane 1: negative sera with the 36 kDa. Lane 2: negative sera with the 36 kDa. Lane 3: negative sera with the 36. Lane 4: negative sera. Lane 5: negative sera. Lane 6: negative sera with the 36 kDa. Lane 7: negative sera with the 36 and 33 kDa. Lane 8: negative sera with the 33 and 21 kDa. Lane 9: positive sera with the 36, 33, and 21 kDa. Lane 10: positive sera with the 98, 36, 33, 32, and 21 kDa.

Table 1.Intrinsic characteristics of the 5 relevant bands (98, 36, 33, 32, and 21 kDa) selected in our home-made immunoblot test
Table 1.
|
Bands kDa |
Positive sera (n = 154) |
Negative sera (n = 100) |
Sensitivity CI 95%
|
Specificity CI 95%
|
PPV CI 95%
|
NPV CI 95%
|
YI |
|
98 |
128 |
13 |
83.1 (76.1, 88.5) |
87.0 (78.4, 92.6) |
90.8 (84.4, 94.8) |
77.0 (67.9, 84.2) |
0.701 |
|
36 |
146 |
11 |
94.8 (89.7, 97.6) |
89.0 (80.8, 94.1) |
93.0 (87.5, 96.3) |
91.8 (83.9, 96.1) |
0.838 |
|
33 |
112 |
7 |
72.7 (64.9, 79.4) |
93.0 (85.6, 96.9) |
94.1 (87.8, 97.4) |
68.9 (60.3, 76.4) |
0.657 |
|
32 |
144 |
8 |
93.5 (88.1, 96.7) |
92.0 (84.4, 96.2) |
94.7 (89.5, 97.5) |
90.2 (82.3, 94.9) |
0.855 |
|
21 |
125 |
12 |
81.2 (73.9, 86.8) |
88.0 (79.6, 93.4) |
91.2 (84.9, 95.2) |
75.2 (66.2, 82.5) |
0.692 |
Table 2.Intrinsic characteristics of the different profiles obtained with the 5 relevant bands (98, 36, 33, 32, and 21 kDa) detected in our immunoblot test
Table 2.
|
Positive sera (n = 154) |
Negative sera (n = 100) |
Sensitivity (CI 95%) |
Specificity (CI 95%) |
PPV (CI 95%) |
NPV (CI 95%) |
YI |
|
Presence of at least 1 band |
154 |
36 |
100.0 (97.0, 100.0) |
64.0 (53.7, 73.2) |
81.1 (74.0, 86.2) |
100.0 (92.9, 100.0) |
0.640 |
|
Presence of at least 2 bands |
153 |
14 |
99.4 (95.9, 100.0) |
86.0 (77.3, 91.9) |
91.6 (86.1, 95.2) |
98.9 (92.9, 99.9) |
0.854 |
|
Presence of a least 3 bands |
150 |
1 |
97.4 (93.1, 99.2) |
99.0 (93.8, 99.9) |
99.3 (95.8, 100.0) |
96.1 (89.8, 98.7) |
0.964 |
|
Presence of a least 4 bands |
129 |
0 |
83.8 (76.8, 89.0) |
100.0 (95.4, 100.0) |
100.0 (96.4, 100.0) |
80.0 (71.7, 86.4) |
0.838 |
|
Simultaneous presence of 5 bands |
69 |
0 |
44.8 (36.9, 53.0) |
100.0 (93.4, 100.0) |
100.0 (93.4, 100.0) |
54.1 (46.6, 61.3) |
0.448 |