Abstract
Trichomonas vaginalis secretes a number of proteases which are suspected to be the cause of pathogenesis; however, little is understood how they manipulate host cells. The mammalian target of rapamycin (mTOR) regulates cell growth, cell proliferation, cell motility, cell survival, protein synthesis, and transcription. We detected various types of metalloproteinases including GP63 protein from T. vaginalis trophozoites, and T. vaginalis GP63 metalloproteinase was confirmed by sequencing and western blot. When SiHa cells were stimulated with live T. vaginalis, T. vaginalis excretory-secretory products (ESP) or T. vaginalis lysate, live T. vaginalis and T. vaginalis ESP induced the mTOR cleavage in both time- and parasite load-dependent manner, but T. vaginalis lysate did not. Pretreatment of T. vaginalis with a metalloproteinase inhibitor, 1,10-phenanthroline, completely disappeared the mTOR cleavage in SiHa cells. Collectively, T. vaginalis metallopeptidase induces host cell mTOR cleavage, which may be related to survival of the parasite.
-
Key words: Trichomonas vaginalis, 1,10-phenanthroline, mTOR cleavage, SiHa cell, metalloproteinase
INTRODUCTION
Trichomonas vaginalis is one of the major agents of non-viral sexually transmitted diseases in humans. Over 180 million people worldwide are annually infected by this parasite [
1].
T. vaginalis commonly causes vaginitis and perhaps cervicitis in women as well as urethritis in both sexes [
2]. Trichomoniasis also has been associated with premature membrane rupture, preterm delivery, low birth weight, preterm delivery, atypical pelvic inflammatory disease, infertility, predisposition to developing invasive cervical cancer, and increased susceptibility to HIV infection [
1,
3].
T. vaginalis is an extracellular protozoan parasite, thus it needs protease in order to hydrolyze organic components from the mucosa and extracellular matrix of its host cells into glucose, which is then transported into the parasitic cells [
4].
T. vaginalis produces several kinds of proteases such as cysteine proteases, serine proteases, and metalloproteinases, which are important regulators of pathogenesis, invasion, and survival [
4,
5,
6,
7]. GP63 metalloproteinases are the major pathogenic agent of
Leishmania spp.,
Trypanosome cruzi, and
Trypanosome brucei, and play an important role in host-parasite interactions [
8,
9]. There are 48 members of the GP63 protease family in
T. vaginalis [
6]; however, the roles of
T. vaginalis metallpoteinases are not well known until now.
The mammalian target of rapamycin (mTOR) is a serine/threonine protein kinase that regulates cell growth, cell proliferation, cell motility, cell survival, protein synthesis, and transcription [
10]. mTOR is constitutively activated in the presence of growth factor and nutrients, and up-regulation of mTOR can be related to activation of AKT anabolism [
11,
12]. A recent study has shown that the
Leishmania protease GP63 cleaves the mTOR, and then mTOR cleavage results in the inhibition of mTOR complex 1 (mTORC1) and concomitant activation of 4E-BP1 to promote
Leishmania proliferation [
13], because synthesis of type I IFN (IFN-α and IFN-β) is regulated by mTORC1 and its downstream targets p70S6K1/2 [
14].
T. vaginalis has abundant proteases, and phosphatidylinositol 3-kinase (PI3K)/protein kinase-B (AKT)/mTOR/S6K1 signaling is important in regulation of cell proliferation, differentiation, and cellular metabolism [
15].
However, little is known about how T. vaginalis lives as a parasite and regulates host cell protein synthesis, which is considered to be the ultimate consequence of pathogenesis and effect of T. vaginalis infection on host protein synthesis. In the present study, we detected the presence of various types of metalloproteinases in T. vaginalis tachyzoites and elucidated the effect of T. vaginalis metalloproteinase on host cell mTOR in AKT/mTOR/S6K pathway. Thus, human cervical tumor cells (SiHa cells) were stimulated with live T. vaginalis, T. vaginalis excretory-secretory products (ESP) or T. vaginalis lysate, and then examined the post-translational modifications of mTOR, AKT, and p70S6K using western blot. In addition, we used the metallopeptidase inhibitor, 1,10-phenanthroline (1,10-PT), to confirm the roles of T. vaginalis metalloproteinases about the cleavage function of mTOR.
MATERIALS AND METHODS
T. vaginalis culture
The T. vaginalis T016 isolate was cultured in a glass, screw-capped tube containing Diamond's trypticase-yeast extract-maltose (TYM) medium (NAPCO, Winchester, Virginia, USA) supplemented with 10% heat-inactivated horse serum (Sigma-Aldrich, St. Louis, Missouri, USA) in 5% CO2 at 37℃ for 24 hr. Cultured parasites were monitored for motility, and the viability of T. vaginalis was determined before each experiment using trypan blue staining (>99%).
Reverse transcription-PCR (RT-PCR) assay
Total RNA was extracted from
T. vaginalis T016 isolate using Trizol Reagent (Invitrogen, Carlsbad, California, USA), and RNA concentrations were determined spectrophotometrically. RNA (1 µg) was reverse transcribed using the ReverTra Ace RT kit (Toyobo, Osaka, Japan). Following reverse transcription, cDNA was amplified by PCR using Ex Taq (Takara, Tokyo, Japan) under the following conditions: 5 min at 94℃, followed by 30 cycles of 30 sec at 98℃, 30 sec at 57℃, and 1 min at 72℃. The final elongation step was continued for 10 min at 72℃. The primer sequences were used as follows: GP63, MG aminopeptidase P-like metallopeptidase (MP50), M41 FtsH endopeptidase-like metallopeptidase (TVAG_243780.2), oligopeptidase A-like metallopeptidase (TVAG_477180), zinc carboxypeptidase-like metallopeptidase (TVAG_075740), and aspartyl aminopeptidase-like metallopeptidase (TVAG_392410) (
Table 1). PCR products were electrophoresed through a 2% agarose gel and visualized with RedSafe (iNtRON Biotechnology, Seoul, Korea).
To construct pET32a-GP63 expression plasmids, the coding sequence of the T. vaginalis GP63 (GenBank accession no. GU356538.1) were amplified by PCR using Pfu-X DNA Polymerase (Solgent, Daejeon, Korea) from cDNA of T. vaginalis T016 with a pair of oligonucleotide primers: GP63 forward, 5'-CGCGGATCCATGATATTGTCTCTACTCCTCGTTG-3' and reverse, 5'-CCCAAGCTTAATCGATAAATTTGCATCAGGTTCT-3' (recognition sites for BamHI and HindIII, respectively are underlined). The PCR products were digested with the appropriate restriction enzyme and cloned into the BamHI/HindIII sites of the pET-32a (+) expression vector with N-terminal His-tag. The resulting plasmids were named pET32a-GP63. Recombinant plasmids were confirmed by restriction analysis and PCR sequencing (Solgent, Daejeon, South Korea). His-tagged GP63 protein was induced by 1 mM IPTG at 37℃ for 3 hr in BL21 (DE3) Escherichia coli. Finally, GP63 proteins in the supernatant were separated by 10% SDS-PAGE and visualized by Coomassie blue staining or detected by western blotting using the anti-His antibody.
Preparation of T. vaginalis lysate
To prepare T. vaginalis lysates, T. vaginalis were harvested in the logarithmic phase and washed 3 times in PBS (pH 6.2). T. vaginalis pellets were resuspended in PBS and lysed using a sonicator, and then centrifuged at 10,000 g for 30 min. The supernatant was collected and used as T. vaginalis lysate. The lysate was filtered through a 0.2-µm-pore-size filter and stored at -70℃ until required. The T. vaginalis lysate concentration was determined by the Bradford assay using bovine serum albumin (BSA) as the standard and stored at -70℃ until use.
Culture of SiHa cells
Human cervical cancer cell lines, SiHa cells, were obtained from the American Type Culture Collection (ATCC, Manassas, Virginia, USA) and maintained in Dulbecco's Modified Eagle's Medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum (FBS; Gibco BRL, Grand Island, New York, USA) and antibiotic-antimycotic (Gibco BRL) in a 5% CO2 at 37℃.
Stimulation of SiHa cells with live T. vaginalis, T. vaginalis ESP, or T. vaginalis lysate
SiHa cell monolayers (1×106) were washed with PBS (pH 7.4) and live T. vaginalis were incubated with SiHa cell monolyers in the medium mixture (DMEM/TYM=2:1) at a multiplicity of infection (MOI) of 2 for 16 hr. To investigate whether T. vaginalis ESP were critical for SiHa cell protein synthesis, we used the 6-well Transwell permeable supports system (Costar, Cambridge, Massachusetts, USA). These inserts, which have a porous (pore diameter 3 µm) membrane, serve as the upper chambers and ordinary tissue culture plate wells serve as the lower chambers. SiHa cells (1×106) was added to the lower chamber and medium containing T. vaginalis (2×106) was added to the upper chambers and the plates were then incubated for 16 hr. Otherwise, SiHa cells were treated with T. vaginalis lysate (100 µg/ml) for 16 hr.
To evaluate the effect of mTOR according to the incubation time and parasite load, SiHa cells were incubated with live T. vaginalis at MOI 2 for 0.5, 1, 2, 4, 8, 16 hr, or SiHa cells were infected with live T. vaginalis at MOI 0.5, 1, and 2 for 2 hr. At the indicated time points, cells were collected and washed in PBS.
Treatment of 1,10-PT-pretreated T. vaginalis
To determine whether T. vaginalis metalloproteinases are involved in the cleavage host mTOR, T. vaginalis (MOI 2) were treated for 30 min with 5 mM 1,10-phenanthroline (1,10-PT; Sigma-Aldrich) and the control group T. vaginalis were mock-treated in PBS. Then, T. vaginalis were washed with PBS and infected with SiHa cells for 16 hr.
Western blot analysis
Proteins were extracted using the PRO-PREP Protein Extraction Solution (iNtRON Biotechnology, Seoul, Korea) supplemented with a complete cocktail of protease inhibitors (Roche, Basel, Switzerland) for 15 min on ice. After centrifugation at 14,000 g for 15 min at 4℃, the supernatant was collected, and equal volumes of protein from each sample were separated by SDS-PAGE and transferred to a polyvinylidene difluoride membrane. The membranes were blocked in Tris-buffered saline (20 mM Tris, 137 mM NaCl, pH 7.6) containing 0.1% Tween-20 (TBST) and 5% skim milk. After being washed once in TBST, membranes were incubated overnight at 4℃ with the primary antibodies diluted in TBST supplemented with 5% BSA. The antibodies used included rabbit polyclonal anti-mTOR, rabbit polyclonal anti-AKT, rabbit polyclonal anti-p70S6K (all from Cell Signaling Technology Inc., Beverly, Massachusetts, USA), and mouse monoclonal anti-α tubulin (Santa Cruz Biotechnology, Santa Cruz, California, USA). Following 3 consecutive washes in TBST, membranes were incubated for 90 min with horseradish peroxidase-conjugated anti-rabbit or anti-mouse IgG (Santa Cruz Biotechnology) diluted 1:10,000 with incubation buffer, as described above. After extensive washing, bound secondary antibodies were visualized using an enhanced ECL chemiluminescence detection kit (GE Healthcare, Little Chalfont, UK).
RESULTS
Identification of various T. vaginalis metalloproteinases
Previous studies demonstrated that metalloproteinase is the largest surface protease family and the second largest surface protein family in
T. vaginalis [
5], and some of these molecules may be involved in the damage to the host [
16]. To better define the
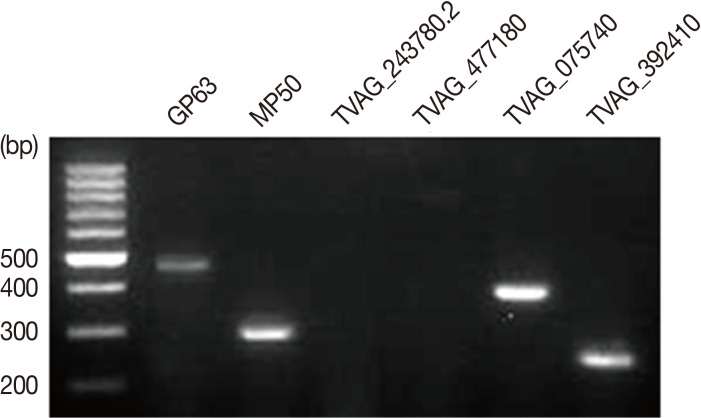
T. vaginalis T016 isolate metalloproteinases, GP63, MP50, TVAG_243780.2, TVAG_477180, TVAG_075740, and TVAG_392410 expression levels were detected by RT-PCR. Interestingly, GP63 (501 bp), MP50 (304 bp), TVAG_075740 (400 bp), and TVAG_392410 (249 bp) mRNA expression were detected. However, we could not detect any TVAG_243780.2 and TVAG_477180 mRNA (
Fig. 1).
We constructed the recombinant plasmid pET32a-GP63, and GP63 fragment was sequenced along both strands and compared to GenBank sequences (accession no. GU356538.1). As shown in
Fig. 2, when the complete GP63 region nucleotide sequences were compared with the GU356538.1 sequences, they were 99.6% base pare identities. In the 1,893 bp of open reading frame, there were only 7 nucleotide differences.
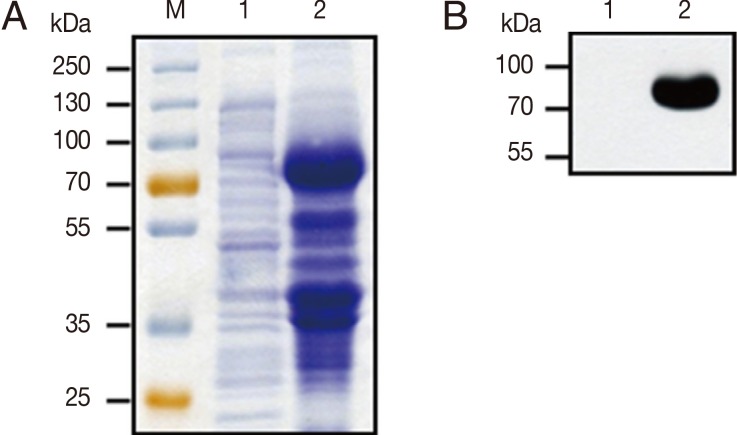
To identify the expression level of GP63 protein, IPTG-induced recombinant His-tagged GP63 protein bands were separated by 10% SDS-PAGE and visualized by Coomassie blue staining (
Fig. 3A). The lysate of IPTG-induced pET32a-GP63 BL21(DE3)
E. coli showed the specific high expression of 89 kDa His-tagged GP63 protein. However, uninduced pET32a-GP63 transfomed
E. coli lysate did not expresse 89 kDa GP63 protein. Further, His-tagged GP63 protein band was also confirmed by western blotting using the anti-His antibody (
Fig. 3B). These results clearly indicate that
T. vaginalis metalloproteinase GP63 recombinant protein can highly expressed in vitro using a bacterial expression system.
The AKT/mTOR/p70S6K pathway regulates cell growth and proliferation via the regulation of protein synthesis [
17]. To investigate the effect of
T. vaginalis infection in the AKT/mTOR/p70S6K pathway, SiHa cells were incubated with live
T. vaginalis,
T. vaginalis ESP (transwell inside the live
T. vaginalis), or
T. vaginalis lysate for 16 hr, and then the posttranslational modification of mTOR, AKT, and p70S6K were observed by western blot. As shown in
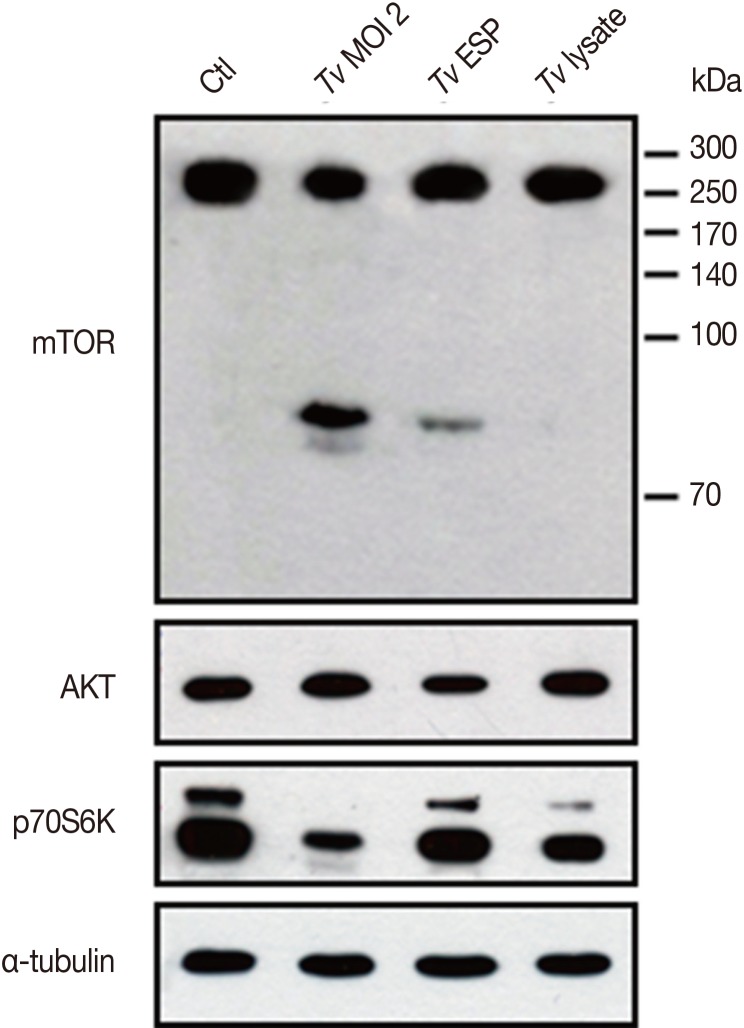
Fig. 4, live
T. vaginalis and
T. vaginalis ESP induced cleavage of host cell mTOR to generate approximately 85 kDa smaller protein band and the live
T. vaginalis induced higher levels of mTOR cleavage than
T. vaginalis ESP that even smaller band approximately 80 kDa appeared right beneath the 85 kDa band. Based on the reciprocal band intensity change between original larger mTOR band and cleaved smaller band, comparison of the mTOR-immunoreactive protein band sizes and the epitope (Ser2481 containing polypeptide) information for the antibody what we used, it is presumed that the cleavage of mTOR occurs at the N-terminal region of mTOR by
T. vaginalis. Interestingly, AKT protein level was not affected by
T. vaginalis but mTOR downstream target p70S6K had suffered degradation or cleavage by live
T. vaginalis infection.
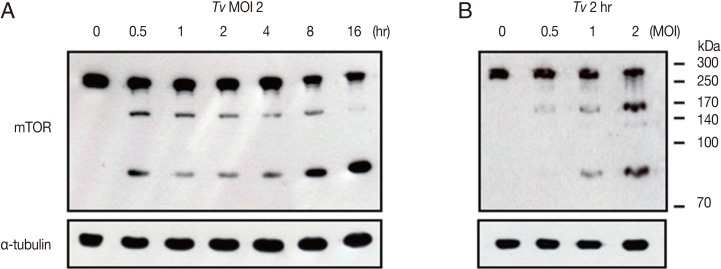
In order to examine how fast
T. vaginalis infection makes effect on host cells, we performed time-course studies of mTOR cleavage after incubation of SiHa cells with live
T. vaginalis for 0.5, 1, 2, 4, 8, and 16 hr. As shown in
Fig. 5,
T. vaginalis induced the cleavage of SiHa cell mTOR from 30 min after infection, and these cleavage phenomena were time-dependent, and prominently high levels of cleaved mTOR were detected after infection with
T. vaginalis at 8 hr and 16 hr (
Fig. 5A). Similarly, when the SiHa cells were infected with
T. vaginalis at MOI 0.5, 1, and 2 for 2 hr,
T. vaginalis induced mTOR cleavage in parasite load-dependent manner. A high level of cleaved mTOR was also detected in SiHa cells at
T. vaginalis MOI 2 for 2 hr (
Fig. 5B). Interestingly,
Fig. 5A and 5B shows that, based on the infection time and infection strength, mTOR cleavage occurs in more than 2 phases, and in early infection, first N-terminal cleavage occurs to generate rather intermediate form of 170 kDa mTOR fragment. Then, as infection time elongates, this 170 kDa fragments get further cleaved to generate 85 kDa fragment of mTOR, and after 16 hr, most of 170 kDa intermediate mTOR fragment disappeared, and the band intensity of 85 kDa mTOR fragment become stronger.
To determine whether the
T. vaginalis metallopeptidase plays a vital role in cleavage of SiHa cell mTOR, live
T. vaginalis (MOI 2) were treated for 30 min with 5 mM metallopeptidase inhibitor, 1,10-PT. After incubation finished,
T. vaginalis was washed with PBS to remove the inhibitor from the surface of parasites, and then infected with SiHa cells for 16 hr, and cell morphology was observed by phase-contrast light microscopy. Some of live
T. vaginalis-infected SiHa cells detached from the culture dish and were slightly darker than the control group (
Fig. 6B, C). In addition, cells infected with 1,10-PT-pretreated
T. vaginalis lost their lethal effect on cell survival, so that the infected host cells were morphologically similar with the control group (
Fig. 6C). Our experimental results showed that the metallopeptidase inhibitor, 1,10-PT, was found to inhibit the mTOR cleavage significantly in SiHa cells (
Fig. 6D). The 170 kDa and 85 kDa mTOR fragment bands completely disappeared by 1,10-PT pretreatment and regained the correct immune positive band size of intact mTOR protein. This is the first report about that the
T. vaginalis metallopeptidase plays a vital role in cleavage of host cell mTOR.
DISCUSSION
It was known that
T. vaginalis proteases are important regulators of pathogenesis, invasion, and survival [
5,
6], and
T. vaginalis genome contains 13 families of metallopeptidases [
5]. From this study, we tried to confirm whether
T. vaginalis metalloproteinases are involved in host cell mTOR cleavage or not. In the present study, we detected several kinds of metalloproteinases in live
T. vaginalis parasites. We also constructed pET32a-GP63 expression plasmids, and confirmed this His-tagged GP63 fusion protein was highly expressed. Furthermore, 1,10-PT-pretreated
T. vaginalis did not induce SiHa cell mTOR cleavage. These findings indicate that
T. vaginalis metalloproteinases are involved in mTOR cleavage, and when this activity is inhibited by 1,10-PT,
T. vaginalis cannot induce SiHa cell mTOR cleavage.
The mTOR acts as a 'master switch' of cellular catabolism and anabolism, signaling cells to expand, grow, and proliferate [
18]. Disruption of the AKT/mTOR pathway by
L. major is dependent on the surface metalloprotease GP63 [
18], and the
Leishmania metalloprotease GP63 also cleaves the mTOR [
13].
Leishmania metallopeptidase has been found to be involved not only in the cleavage and degradation of various kinases and transcription factors, but also to be the major molecule modulating host negative regulatory mechanisms [
20]. In the present study, we confirmed that
T. vaginalis contains zinc metallopeptidase with a zinc-binding motif of HEXXH (GP63), MG aminopeptidase P-like metallopeptidase (MP50), M41 FtsH endopeptidase-like metallopeptidase (TVAG_243780.2), oligopeptidase A-like metallopeptidase (TVAG_477180), Zinc carboxypeptidase-like metallopeptidase (TVAG_075740), and aspartyl aminopeptidase-like metallopeptidase (TVAG_392410).
T. vaginalis metalloproteinase is the largest surface protease family and the second largest surface protein family in
T. vaginalis [
5]; however, there was no report about the effects of mTOR in
T. vaginalis-infected cells. We report here another case of host cell mTOR cleavage induced by live
T. vaginalis and
T. vaginalis ESP, except
Leishmania infection. The host cell mTOR was cleaved as early as 30 min after
T. vaginalis infection, and this mTOR cleavage occurred as time- and parasite burden-dependent manner. It may be important to understand the host-parasite relationship in
T. vaginalis-infected SiHa cells. We need further studies to find out the mechanisms of mTOR cleavage by
T. vaginalis.
PI3K/AKT/mTOR pathway plays an important role in cellular growth and survival [
20]. The mTOR cleavage by
Leishmania GP63 metalloproteinase inhibits host cell translation initiation, thus parasites survive and induce the pathogenesis of leishmaniasis [
13]. From this study, we confirmed that
T. vaginalis has various kinds of metalloproteinases by RT-PCR, sequencing analysis and western blot.
T. vaginalis induced the SiHa cell mTOR cleavage as well as decreased the protein levels of p70S6K protein levels in comparison to uninfected control group. These data suggest that SiHa cell mTOR cleavage by
T. vaginalis may induce the inhibition of host cell protein synthesis, parasite establishes host infection, and starts proliferation, which is a similar mechanism of
Leishmania for its growth [
13]. Base on the previous paper,
T. vaginalis is strong inducer of host cell apoptosis [
7]. It can be explained that host cell apoptosis by
T. vaginalis could be related with disruption of PI3K/AKT/mTOR pathway according to our results and previous papers [
22,
23]. Namely, suppression of PI3K/AKT/mTOR/S6K pathway by β-caryophyllene oxide could facilitate downregulation of various cell survival genes and lead to apoptosis in tumor cells [
22], and
M. citriodora and its major constituent thymol inhibit the PI3K/AKT/mTOR pathway and induce apoptosis via both extrinsic and intrinsic pathways in human leukemia HL-60 cells [
23].
Taken together, our results indicate that T. vaginalis metallopeptidase plays a vital role in cleavage of host cell mTOR by disruption of mTOR/p70S6K pathway, and T. vaginalis cleaves host cell mTOR probably to block various anti-parasitic events of its host which are under mTOR control and make profitable cellular environment for its survival. These findings raise an interesting possibility that agents that block mTOR cleavage could be therapeutically useful as an approach for treatment of trichomoniasis. Further investigation will shed light on the molecular mechanisms of mTOR cleavage by T. vaginalis GP63 and disruption of mTOR/p70S6K pathway.
Medical Scientific Research Foundation of Guangdong ProvinceB2013309
National Research Foundation of Korea2007-0054932
Notes
-
We have no conflict of interest related to this study.
ACKNOWLEDGMENTS
This study was supported by grants from the Medical Scientific Research Foundation of Guangdong Province, China (grant no. B2013309 to Juan-Hua Quan), and the National Research Foundation of Korea (NRF) grant funded by the Korea Government (MSIP) (no. 2007-0054932).
References
- 1. Van der Pol B. Trichomonas vaginalis infection: the most prevalent nonviral sexually transmitted infection receives the least public health attention. Clin Infect Dis 2007;44:23-25.
- 2. Gómez-Barrio A, Nogal-Ruiz JJ, Montero-Pereira D, Rodríguez-Gallego E, Romero-Fernández E, Escario JA. Biological variability in clinical isolates of Trichomonas vaginalis. Mem Inst Oswaldo Cruz 2002;97:893-896.
- 3. Poole DN, McClelland RS. Global epidemiology of Trichomonas vaginalis. Sex Transm Infect 2013;89:418-422.
- 4. Arroyo R, Alderete JF. Trichomonas vaginalis surface proteinase activity is necessary for parasite adherence to epithelial cells. Infect Immun 1989;57:2991-2997.
- 5. Hirt RP, Noel CJ, Sicheritz-Ponten T, Tachezy J, Fiori PL. Trichomonas vaginalis surface proteins: a view from the genome. Trends Parasitol 2007;23:540-547.
- 6. Ma L, Meng Q, Cheng W, Sung Y, Tang P, Hu S, Yu J. Involvement of the GP63 protease in infection of Trichomonas vaginalis. Parasitol Res 2011;109:71-79.
- 7. Sommer U, Costello CE, Hayes GR, Beach DH, Gilbert RO, Lucas JJ, Singh BN. Identification of Trichomonas vaginalis cysteine proteases that induce apoptosis in human vaginal epithelial cells. J Biol Chem 2005;280:23853-23860.
- 8. Kulkarni MM, Olson CL, Engman DM, McGwire BS. Trypanosoma cruzi GP63 proteins undergo stage-specific differential posttranslational modification and are important for host cell infection. Infect Immun 2009;77:2193-2200.
- 9. Joshi PB, Kelly BL, Kamhawi S, Sacks DL, McMaster WR. Targeted gene deletion in Leishmania major identifies leishmanolysin (GP63) as a virulence factor. Mol Biochem Parasitol 2002;120:33-40.
- 10. Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev 2004;18:1926-1945.
- 11. Dennis PB, Jaeschke A, Saitoh M, Fowler B, Kozma SC, Thomas G. Mammalian TOR: a homeostatic ATP sensor. Science 2001;294:1102-1105.
- 12. Edinger AL, Thompson CB. Akt maintains cell size and survival by increasing mTOR-dependent nutrient uptake. Mol Biol Cell 2002;13:2276-2288.
- 13. Jaramillo M, Gomez MA, Larsson O, Shio MT, Topisirovic I, Contreras I, Luxenburg R, Rosenfeld A, Colina R, McMaster RW, Olivier M, Costa-Mattioli M, Sonenberg N. Leishmania repression of host translation through mTOR cleavage is required for parasite survival and infection. Cell Host Microbe 2011;9:331-341.
- 14. Cao W, Manicassamy S, Tang H, Kasturi SP, Pirani A, Murthy N, Pulendran B. Toll-like receptor-mediated induction of type I interferon in plasmacytoid dendritic cells requires the rapamycin-sensitive PI(3)K-mTOR-p70S6K pathway. Nat Immunol 2008;9:1157-1164.
- 15. Polivka J Jr, Janku F. Molecular targets for cancer therapy in the PI3K/AKT/mTOR pathway. Pharmacol Ther 2014;142:164-175.
- 16. Alvarez-Sánchez ME, Avila-González L, Becerril-García C, Fattel-Facenda LV, Ortega-López J, Arroyo R. A novel cysteine proteinase (CP65) of Trichomonas vaginalis involved in cytotoxicity. Microb Pathog 2000;28:193-202.
- 17. Galbaugh T, Cerrito MG, Jose CC, Cutler ML. EGF-induced activation of Akt results in mTOR-dependent p70S6 kinase phosphorylation and inhibition of HC11 cell lactogenic differentiation. BMC Cell Biol 2006;7:34.
- 18. Vignot S, Faivre S, Aguirre D, Raymond E. mTOR-targeted therapy of cancer with rapamycin derivatives. Ann Oncol 2005;16:525-537.
- 19. Matte C, Descoteaux A. Disruption of the AKT/mTOR pathway by Leishmania major promastigotes. BMC Proceedings 2011;5(suppl 1):44.
- 20. Isnard A, Shio MT, Olivier M. Impact of Leishmania metalloprotease GP63 on macrophage signaling. Front Cell Infect Microbiol 2012;2:72.
- 21. Slomovitz BM, Coleman RL. The PI3K/AKT/mTOR pathway as a therapeutic target in endometrial cancer. Clin Cancer Res 2012;18:5856-5864.
- 22. Park KR, Nam D, Yun HM, Lee SG, Jang HJ, Sethi G, Cho SK, Ahn KS. β-caryophyllene oxide inhibits growth and induces apoptosis through the suppression of PI3K/AKT/mTOR/S6K1 pathways and ROS-mediated MAPKs activation. Cancer Lett 2011;312:178-188.
- 23. Pathania AS, Guru SK, Verma MK, Sharma C, Abdullah ST, Malik F, Chandra S, Katoch M, Bhushan S. Disruption of the PI3K/AKT/mTOR signaling cascade and induction of apoptosis in HL-60 cells by an essential oil from Monarda citriodora. Food Chem Toxicol 2013;62:246-254.
Fig. 1
T. vaginalis T016 isolate expressing several metalloproteinases. GP63 (501 bp), MP50 (304 bp), TVAG_243780.2 (598 bp), TVAG_477180 (800 bp), TVAG_075740 (400 bp), and TVAG_392410 (249 bp) metalloproteinase mRNA expression levels were detected by RT-PCR.

Fig. 2The GP63 nucleotide sequences of recombinant plasmid pET32a-GP63 were compared with GenBank sequence (accession no. GU356538.1). Base homologies were indicated with blue, and base changes were shown in white. The total length of T. vaginalis T016 isolate GP63 region is 1,893 bp.

Fig. 3Expression and identification of recombinant proteins. (A) Proteins were submitted to SDS-PAGE and revealed by Coomassie blue staining. M, protein molecular size marker; lane 1, pET32a-GP63 transfomed E. coli; lane 2, IPTG-induced pET32a-GP63 transfomed E. coli. (B) Western blot analysis of E. coli lysate with anti-His primary antibody. Lane 1, pET32a-GP63 transfomed E. coli lysate; lane 2, IPTG-induced pET32a-GP63 transfomed E. coli lysate.

Fig. 4Live T. vaginalis and T. vaginalis ESP induced cleavage of host cell mTOR. After SiHa cells were incubated with live T. vaginalis at a multiplicity of infection (MOI) 2, T. vaginalis ESP (transwell) or T. vaginalis lysate (100 µg/ml) for 16 hr, protein levels of mTOR, AKT, and p70S6K were shown by western blot. Anti-α-tubulin antibodies were employed to confirm equal loading of the cell extracts. A representative result of 3 independent replicates is shown.

Fig. 5
T. vaginalis-induced cleavage of host cell mTOR is time- and parasite load-dependent manner. After SiHa cells were incubated with (A) T. vaginalis at MOI 2 for the indicated time points or (B) SiHa cells were infected with T. vaginalis at MOI 0.5, 1, and 2 for 2 hr, cells were collected, and levels of mTOR was measured. Anti-α-tubulin antibodies were employed to confirm equal loading of the cell extracts. A representative result of 3 independent replicates is shown.

Fig. 6Cleavage of host cell mTOR induced by T. vaginalis was inhibited with metallopeptidase inhibitor, 1,10-phenanthroline (1,10-PT). T. vaginalis (MOI 2) were treated for 30 min with 1,10-PT (5 mM), and the control group T. vaginalis were mock-treated in PBS, and then T. vaginalis were washed with PBS and infected with SiHa cells. After 16 hr later, (A-C) cells were visualized under phage-contrast microscope, and (D) western blot was performed in order to detect mTOR. Anti-α-tubulin antibodies were employed to confirm equal loading of the cell extracts. A representative result of 3 independent replicates is shown. *, SiHa cell; #, trophozoite of T. vaginalis.

Table 1.Primers used for detection of various kinds of metalloproteinases from Trichomonas vaginalis
Table 1.
|
Gene |
|
Primer sequence |
GenBank accession no. |
PCR product size (bp) |
|
GP63 |
(F) |
ACGCTGTCCTTGCAATTCTT |
GU356538.1 |
501 |
|
(R) |
TTGCGTTTTCTTTTGTGCAT |
|
|
|
MP50 |
(F) |
TCTCGACTGCGGATTCTTCT |
JF263458.1 |
304 |
|
(R) |
TCCGACGTGATGAGTCAAAC |
|
|
|
TVAG_243780.2 |
(F) |
TGCAACAAATAGGGCAGACA |
KF269491.1 |
598 |
|
(R) |
AGCCCTCTCAATGTCGTTTG |
|
|
|
TVAG_477180 |
(F) |
TGTCCTCAAACCATGGAACA |
XM_001583725.1 |
800 |
|
(R) |
ATTTGTCGGCAACAACATCA |
|
|
|
TVAG_075740 |
(F) |
ATTCCCTGAGTGGGCTTTTT |
XM_001583763.1 |
400 |
|
(R) |
TGGATGCGTACGAGCAACTA |
|
|
|
TVAG_392410 |
(F) |
GTCGCATCAGAGCGTATTGA |
XM_001327312.1 |
249 |
|
(R) |
CCATGCGTGAGCATTATCTG |
|
|
|
TVAG_077490 |
(F) |
AGCCAACGAAACAGACTTCC |
XM_001325199.1 |
201 |
|
(R) |
ATGATGTGATGGTGCTTGGA |
|
|
|
TVAG_371040 |
(F) |
CGCCGAGACAAACAGAGAAT |
XM_001300222.1 |
899 |
|
(R) |
GATCAGCACCATGAATCCAA |
|
|