Abstract
Surgery remains the preferred treatment for hydatid cyst (cystic echinococcosis, CE). Various scolicidal agents have been used for inactivation of protoscolices during surgery, but most of them are associated with adverse side effects. The present study aimed to evaluate the in vitro scolicidal effect of Nigella sativa (Ranunculaceae) essential oil and also its active principle, thymoquinone, against protoscolices of hydatid cysts. Protoscolices were aseptically aspirated from sheep livers having hydatid cysts. Various concentrations of the essential oil (0.01-10 mg/ml) and thymoquinone (0.125-1.0 mg/ml) were used for 5 to 60 min. Viability of protoscolices was confirmed by 0.1% eosin staining. Furthermore, the components of the N. sativa essential oil were identified by gas chromatography/mass spectroscopy (GC/MS). Our study revealed that the essential oil of N. sativa at the concentration of 10 mg/ml and its main component, thymoquinone, at the concentration of 1 mg/ml had potent scolicidal activities against protoscolices of Echinococcus granulosus after 10 min exposure. Moreover, thymoquinone (42.4%), p-cymene (14.1%), carvacrol (10.3%), and longifolene (6.1%) were found to be the major components of N. sativa essential oil by GC/MS analysis. The results of this study indicated the potential of N. sativa as a natural source for production of a new scolicidal agent for use in hydatid cyst surgery. However, further studies will be needed to confirm these results by checking the essential oil and its active component in in vivo models.
-
Key words: Echinococcus granulosus, Ranunculaceae, thymoquinone, cystic echinococcosis
INTRODUCTION
Hydatid cyst (cystic echinococcosis, CE), a parasitic infection caused by the larval stage of dog tapeworm
Echinococcus granulosus, has been identified as a major public health and economic problem in developing countries, including Iran [
1]. The dog is the definitive host, where adult tapeworms attached to the intestinal epithelium, and humans as well as domestic livestock can be the intermediate host, where the worms form hydatid cysts in various organs [
2]. At present, surgery remains the preferred treatment for particular WHO stage disease. In addition, chemotherapy with benzimidazoles and PAIR (puncture, aspiration, injection, and reaspiration) are recommended as alternative treatments to surgery, especially for the patients who cannot tolerate surgery [
3,
4]. Furthermore, to reduce the risk of intraoperative spillage of the cyst contents (protoscolices) and subsequently recurrence of CE and secondary infection, which is observed in nearly 10% of the postoperative cases, the use of effective scolicidal agents are essential [
5,
6].
Various chemical scolicidal agents such as hypertonic saline, Ag-nitrate, cetrimide, and ethanol have been used for inactivation of protoscolices during surgery, but most of them are associated with adverse side effects such as sclerosane cholangititis (biliary tract fibrosis), liver necrosis, and methemoglobinemia [
7,
8]. Thus, a rapid and complete scolicidal effect with no local or systemic side effects and also low cost are some properties of an ideal scolicidal solution for hydatid cyst surgery [
9]. Since last centuries, plant extracts and plant-derived compounds due to having less side effects, low cost, and high availability are a successful approach to treat a wide range of diseases, such as infectious diseases.
Nigella sativa L. (family Ranunculaceae) is commonly known as black seed grown in the Middle East, Eastern Europe, and Western and Middle Asia which is traditionally used as a natural treatment for a number of diseases and conditions, including asthma, hypertension, diabetes, inflammation, cough, bronchitis, headache, eczema, fever, dizziness, and influenza [
10]. Various pharmacological effects such as antioxidant, anti-inflammatory, anticancer, antimicrobial have been related to
N. sativa or its active principles which include thymoquinone, carvacrol, ρ-cymene, and thymol [
10,
11,
12]. Moreover, in various studies, antibacterial, antifungal, antiviral, and antiparasitic effects of
N. sativa and its derivatives have been demonstrated [
13,
14,
15,
16]. The aim of the present study was to determine the scolicidal effects of essential oil of
N. sativa and also its active principle, thymoquinone, against protoscolices of hydatid cysts using in vitro model.
MATERIALS AND METHODS
Collection of plant materials
The seeds of N. sativa were collected from rural regions of Bam district of Kerman province, Iran in September 2012. The identity was confirmed by a botanist at the Botany Department of Shahid Bahonar University, Kerman, Iran. A voucher specimen of the plant material was deposited at the Herbarium of Department of Pharmacognosy of School of Pharmacy, Kerman University of Medical Science, Kerman, Iran (KF575).
Isolation of the essential oil
Crushed seeds were extracted with light petroleum (BP 40°-60℃) using a Soxhlet apparatus. The solvent was removed under vacuum and the brownish residue was steam distilled. Extraction of the aqueous distillate with n-hexane and removal of the solvent gave the essential oil. The essential oil was stored in sealed vials at 2-8℃ until testing.
Gas chromatography (GC)/mass spectrometry (MS) analysis of essential oil
GC analysis; GC analysis was carried out by a Hewlett-Packard 6890 (Palo Alto, California, USA) with a HP-5MS column (30 m×0.25 mm, film thickness 0.25 mm). The column temperature was maintained at 60℃ for 3 min and programmed to 220℃ at a rate of 5℃ per min, and kept constant at 220℃ for 5 min. Injector and interface temperatures were 220℃ and 250℃, respectively. The flow rate of helium as the carrier gas was (1 ml/min C.F). The percentages were calculated by electronic integration of FID peak areas without the use of response factors correction. Linear retention indices for all components were determined by coinjection of the samples with a solution containing homologous series of C8-C22 n-alkanes.
GC/MS analysis; GC/MS analysis was performed using a Thermoquest-Finnigan gas chromatograph (Austin, Texas, USA) equipped with fused silica capillary DB-5 column (30 m×0.25 mm, film thickness 0.25 mm) coupled with a TRACE mass (Manchester, UK). Helium was used as the carrier gas with ionization voltage of 70 eV. Ion source and interface temperatures were 220℃ and 250℃, respectively. Mass range was from 40 to 400 u. Oven temperature program was the same given above for the GC.
Identification of the essential oil components
The components of the essential oil were identified by comparison of their relative retention time and mass spectra with those of standards Wiley 2001 library data of the GC/MS system or with those reported in the literature [
17].
Thymoquinone was obtained from Sigma-Aldrich (St. Louis, Missouri, USA), dissolved in the dimethyl sulfoxide (DMSO). Final concentration of DMSO never exceeded 1% either in control or in treated samples.
Drug dilutions
Two hundred mg of thymoquinone dissolved in 4 ml of DMSO and serial dilutions were subsequently made to obtain thymoquinone at concentrations of 0.125, 0.25, 0.5, and 1.0 mg/ml. For the preparation of dilutions of N. sativa essential oil, 0.1 ml of the essential oil was dissolved in 9.7 ml of normal saline. In addition, to enhance the dispersal of the essential oil in normal saline, 0.3 ml of Tween 20 (Sigma-Aldrich) was added to the test tube. The resulting solution was mixed adequately by a magnetic stirrer. Serial dilution was then made to obtain the essential oil at 0.01, 0.1, 1.0, and 10 mg/ml. The selection of dilutions of the thymoquinone and the essential oil of N. sativa was based on initial experiments, which also showed that DMSO below 1.5% had no effect on the growth of protoscolices. In the present investigation, the concentration of DMSO in various dilutions was 1% and below.
Collection of protoscolices
In this study, the protoscolices of E. granulosus were obtained from livers of naturally infected sheep and goats slaughtered at Kerman abattoir, southeastern Iran and carried to the Parasitology Laboratory at the Department of Parasitology and Mycology, Kerman University of Medical Sciences, Kerman, Iran. The hydatid fluid aspirated by a 20 ml syringe and aseptically transferred into a flask was left to set for 30 min for protoscolices to settle down. The supernatant was discarded and the protoscolices were washed 2 times with PBS (pH 7.2) solution. The number of protoscolices per ml was adjusted as 2×103 protoscolices in 0.9% NaCl solution with at least 90% viability rate. The viability of the protoscolices was confirmed by their flame cell motility and impermeability to 0.1% eosin solution under a light microscope.
Effects on protoscolices
In order to evaluate the scolicidal effects of
N. sativa essential oil and its main component, thymoquinone, against protoscolices of hydatid cysts, various concentrations of the essential oil and thymoquinone were used for 5, 10, 20, 30, and 60 min. At first, 0.5 ml of the protoscolices (2×10
3/ml) solution was placed in test tubes. Then, 0.5 ml of various concentrations of the essential oil and thymoquinone were added to each test tube. The contents of the tubes were gently mixed and then incubated at 37℃ for 5, 10, 20, 30, and 60 min. At the end of each incubation time, the upper phase was carefully removed so as not to interrupt the protoscolices. 50 µl of 0.1% eosin stain (Sigma-Aldrich) was then added to the remaining settled protoscolices and mixed gently. The upper portion of the solution was discarded after 10 min of incubation. The remaining pellet of protoscolices was then smeared on a glass slide, covered with a cover glass, and examined under a light microscope. The percentages of dead protoscolices were determined by counting 300 protoscolices [
18]. In addition, normal saline containing Tween 20 and 20% hypertonic saline were used as control groups.
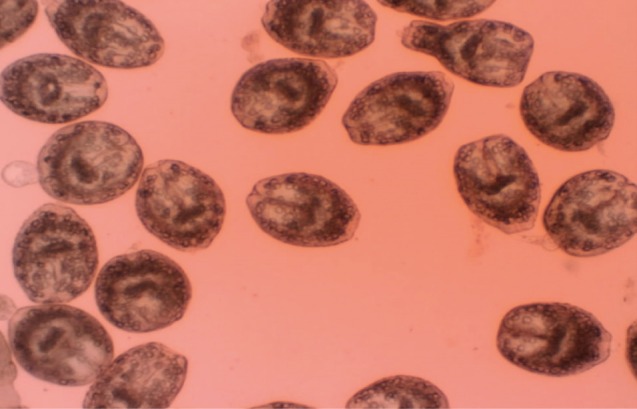
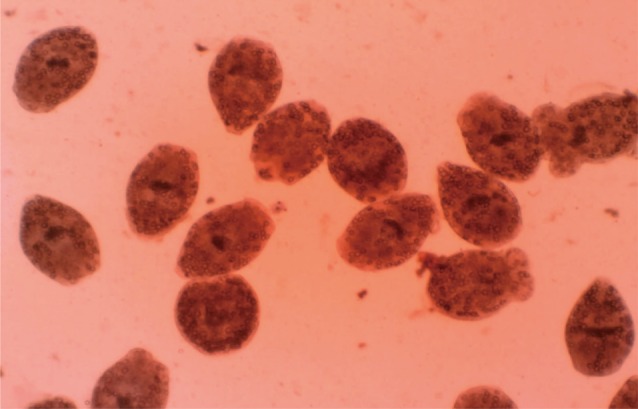
Eosin exclusion test was used to investigate the viability of protoscolices [
19]. Eosin solution with a concentration of 0.1% (1 g of eosin powder in 1,000 ml distilled water) was used. After exposure to the stain, live protoscolices remained colorless and showed characteristic muscular movements and flame cell activity (
Fig. 1), whereas dead protoscolices absorbed eosin and colored red (
Fig. 2).
All the tests were performed in triplicate. Data analysis was carried out by using SPSS statistical package (version 17.0) (SPSS Inc., Chicago, Illinois, USA). Differences between test and control groups were analyzed by the t-test. In addition, P<0.05 was considered statistically significant.
RESULTS
GC-MS analysis of N. sativa essential oil
The essential oil of
N. sativa obtained by Soxhlet extraction was analyzed using GC/MS.
Table 1 shows the identified compounds and percentages obtained by GC/MS. The main components were thymoquinone (42.4%),
p-cymene (14.1%), carvacrol (10.3%), longifolene (6.1%), and 4-terpineol (5.1%).
Scolicidal effects of
N. sativa essential oil at various concentrations following different exposure times are shown in
Table 2. Results indicated that the essential oil of
N. sativa at the concentration of 10 mg/ml after 10 min of exposure killed 100% protoscolices. Similarly, the mean mortality rate of protoscolices after 20 min of exposure to the concentration of 1 mg/ml was 100%. In contrast, the essential oil at the concentration of 0.1 mg/ml killed 8.3, 21.6, 32.3, 55.6, and 76.6%, and at the concentration of 0.01 mg/ml killed 4.6, 14.6, 23.3, 36.3, and 52.1% of the protoscolices after 5, 10, 20, 30, and 60 min of incubation, respectively. As shown in
Table 3, thymoquinone demonstrated more potent scolicidal activity than the essential oil of
N. sativa against protoscolices of
E. granulosus (
P<0.05). The maximum protoscolicidal effect of thymoquinone was found at the concentration of 1 mg/ml, resulting in 100% mortality of protoscolices after 10 min of incubation. Similarly, all protoscolices were killed after 20 min of exposure to the concentration of 0.5 mg/ml of thymoquinone. Moreover, lower concentrations of thymoquinone provoked a delayed protoscolicidal effects, so that 7.3, 17.6, 35.6, 96.3, and 100% of the protoscolices were killed at the concentration of 0.25 mg/ml after 5, 10, 20, 30, and 60 min of applications, respectively (
Table 3). It is of note that all the concentrations of
N. sativa and thymoquinone indicated significant (
P<0.05) scolicidal effects compared to the control group.
DISCUSSION
Since ancient times, plant-derived compounds and plant extracts have been used as a valuable natural resource of traditional remedies [
9]. In the past decades, advent of synthetic antimicrobial drugs caused reluctance in plants as a rich resource for antimicrobial agents [
30]. However, in recent years, emergence of some limitations in the use of these drugs caused changes in situation and interest in the field of ethnobotanical research [
31]. Our findings in this study showed that the essential oil of
N. sativa at the concentration of 10 mg/ml and its main component, thymoquinone, at the concentration of 1 mg/ml had potent scolicidal activities against protoscolices of hydatid cyst after 10 min exposure. These findings were comparable with the scolicidal activity of 20% hypertonic saline (15 min), 20% silver nitrate (20 min), 0.5-1% cetrimide (10 min), H
2O
2 3% (15 min), and 95% ethyl alcohol (15 min). However, an ideal scolicidal agent is defined by its potency at lower concentrations, high efficacy in a shorter time of exposure, stability in the presence of cystic fluid, scolicidal ability inside a cyst, lower toxicity, higher availability, and ability to be prepared rapidly [
32]. In line with our results, several studies have revealed antimicrobial activities of essential oil and various extracts of
N. sativa [
13,
14,
15,
16,
33,
34,
35].
In the present study, we found that the main compound of the
N. sativa essential oil was thymoquinone (42.4%), when analyzed by GC/MS. Similarly, Ali and Blunden [
10] indicated that the most biological activity of
N. sativa seeds is due to thymoquinone. Thus, high scolicidal activity of
N. sativa essential oil could be due to having a higher content of thymoquinone. Since accurate mechanisms of antimicrobial effects of thymoquinone are not clear, further studies are needed to illuminate these mechanisms. However, it has been shown that
N. sativa oil can inhibit DNA synthesis by inhibiting histone deacetylase (HDAC) enzyme interacting with the chromosomes [
36]. In the case of cytotoxic effects of
N. sativa and thymoquinone, El Daly et al. [
37] reported that administration of
N. sativa can reduce the toxic side effects of cisplatin (a widely used chemotherapeutic drug) in rats, including nephrotoxicity. Mahmoud et al. [
38] also showed that thymoquinone has significant cytoprotective properties. Moreover, it has previously been proven that thymoquinone administrated in the drinking water to mice at the concentrations of 0.01, 0.02, and 0.03% for 90 days had no resulting mortality and no signs of toxicity [
39]. Therefore, we can suggest that
N. sativa and its constituents appear to have a low level of toxicity and could be considered safe in used concentrations for hydatid cyst surgery.
To conclude, the results of this study indicated high scolicidal activity of N. sativa essential oil and particularly thymoquinone against protoscolices of hydatid cyst that revealed the potential of N. sativa as a natural source for production of a new scolicidal agent for use in hydatid cyst surgery. However, further studies will be needed to confirm these results by checking the essential oil and its active component in an in vivo model.
Notes
-
The authors declare that there is no conflict of interest in this study.
ACKNOWLEDGMENTS
We would like to thank Dr. Ghasemi Kia for collection of hydatid cysts and Mr. Mir Badiei for data analysis.
References
- 1. Fasihi Harandi M, Budke CM, Rostami S. The monetary burden of cystic echinococcosis in Iran. PLoS Negl Trop Dis 2012;6:e1915.
- 2. Eckert J, Deplazes P. Biological, epidemiological, and clinical aspects of echinococcosis, a zoonosis of increasing concern. Clin Microbiol Rev 2004;17:107-135.
- 3. Brunetti E, Kern P, Vuitton DA. Writing Panel for the WHO-IWGE. Expert consensus for the diagnosis and treatment of cystic and alveolar echinococcosis in humans. Acta Trop 2010;114:1-16.
- 4. Junghanss T, da Silva AM, Horton J, Chiodini PL, Brunetti E. Clinical management of cystic echinococcosis: state of the art, problems, and perspectives. Am J Trop Med Hyg 2008;79:301-311.
- 5. McManus DP, Zhang W, Li J, Bartley PB. Echinococcosis. Lancet 2003;362:1295-1304.
- 6. Besim H, Karayalçin K, Hamamci O, Güngör Ç, Korkmaz A. Scolicidal agents in hydatid cyst surgery. HPB Surg 1998;10:347-351.
- 7. Hosseini SV, Ghanbarzadeh K, Barzin Z, Sadjjadi SM, Tanideh N, Mehrabani D. In vitro protoscolicidal effects of hypertonic glucose on protoscolices of hydatid cyst. Korean J Parasitol 2006;44:239-242.
- 8. Rajabi MA. Fatal reactions and methaemoglobinaemia after silver nitrate irrigation of hydatid cyst. Surg Prac 2009;13:2-7.
- 9. Adas G, Arikan S, Kemik O, Oner A, Sahip N, Karatepe O. Use of albendazole sulfoxide, albendazole sulfone, and combined solutions as scolicidal agents on hydatid cysts (in vitro study). World J Gastroenterol 2009;15:112-116.
- 10. Ali BH, Blunden G. Pharmacological and toxicological properties of Nigella sativa. Phytother Res 2009;17:299-305.
- 11. Randhawa MA, Al-Ghamdi MS. A review of the pharmacotherapeutic effects of Nigella sativa. Pakistan J Med Res 2002;41:77-83.
- 12. Agarwal R, Kharya MD, Shrivastava R. Antimicrobial and anthelmintic activities of the essential oil of Nigella sativa Linn. Indian J Exp Biol 1979;17:1264-1265.
- 13. Mahmoud MR, El-Abhar HS, Saleh S. The effects of Nigella sativa oil against the liver damage induced by Schistosoma mansoni infection in mice. J Ethnopharmacol 2002;79:1-11.
- 14. Khan MA, Ashfaq MK, Zuberi HS, Zuberi AH. The in vivo antifungal activity of the aqueous extract from Nigella sativa seeds. Phytother Res 2003;17:183-186.
- 15. Morsi NM. Antimicrobial effect of crude extracts of Nigella sativa on multiple antibiotics-resistant bacteria. Acta Microbiol Pol 2000;49:63-74.
- 16. Salem ML, Hossain MS. Protective effect of black seed oil from Nigella sativa against murine cytomegalovirus infection. Int J Immunopharmacol 2000;22:729-740.
- 17. Adams RP. Identification of essential oil components by gas chromatography/mass spectroscopy. Carol Stream, Illinois, USA. Allured Publishing Corporation; 2007.
- 18. Moazeni M, Saharkhiz MJ, Hosseini AA. In vitro lethal effect of ajowan (Trachyspermum ammi L.) essential oil on hydatid cyst protoscoleces. Vet Parasitol 2012;187:203-208.
- 19. Smyth JD, Barrett NJ. Procedures for testing the viability of human hydatid cysts following surgical removal, especially after chemotherapy. Trans R Soc Trop Med Hyg 1980;74:649-652.
- 20. Kayaalp C, Balkan M, Aydin C, Ozgurtas T, Tanyuksel M, Kirimlioglu V, Akoglu M, Oner K, Pekcan M. Hypertonic saline in hydatid disease. World J Surg 2001;25:975-979.
- 21. Caglar R, Yuzbasioglu MF, Bulbuloglu E, Gul M, Ezberci F, Kale IT. In vitro effectiveness of different chemical agents on scolices of hydatid cyst. J Invest Surg 2008;21:71-75.
- 22. Erzurumlu K, Hokelek M, Baris S, Sahin M, Birinci A, Amanvermez R, Tac K. Effect of albendazole sulfoxide solution on the scolices and the hepatobiliary system. Eur Surg Res 1998;30:433-438.
- 23. Landa García JI, Alonso E, Gonzalez-Uriarte J, Rodriguez Romano D. Evaluation of scolicidal agents in an experimental hydatid disease model. Eur Surg Res 1997;29:202-208.
- 24. Paksoy Y, Odev K, Sahin M, Arslan A, Koç O. Percutaneous treatment of hydatid cysts: comparison of direct injection of albendazole and hypertonic saline solution. AJR Am J Roentgenol 2005;185:727-734.
- 25. Puryan K, Karadayi K, Topcu O, Canbay E, Sumer Z, Turan M, Karayalcin K, Sen M. Chlorhexidine gluconate: an ideal scolicidal agent in the treatment of intraperitoneal hydatidosis? World J Surg 2005;29:227-230.
- 26. Kilicoglu B, Kismet K, Koru O. Tanyuksel M, Oruc MT, Sorkun K, Akkus MA. The scolicidal effects of honey. Adv Ther 2006;23:1077-1083.
- 27. Mahmoudvand H, Fasihi Harandi M, Shakibaie M, Aflatoonian MR, ZiaAli N, Makki MS, Jahanbakhsh S. Scolicidal effects of biogenic selenium nanoparticles against protoscolices of hydatid cysts. Int J Surg 2014;12:399-403.
- 28. Moazeni M, Nazer A. In vitro effectiveness of garlic (Allium sativum) extract on scolices of hydatid cyst. World J Surg 2010;34:2677-2681.
- 29. Zibaei M, Sarlak A, Delfan B, Ezatpour B, Azargoon A. Scolicidal effects of Olea europaea and Satureja khuzestanica extracts on protoscolices of hydatid cysts. Korean J Parasitol 2012;50:53-56.
- 30. Cowan MM. Plant products as antimicrobial agents. Clin Microbiol Rev 1999;12:564-582.
- 31. McCutcheon AR, Ellis SM, Hancock REW, Tower GH. Antibiotic screening of medicinal plants of the British Columbian native peoples. J Ethnopharmacol 1992;37:213-223.
- 32. World Health Organization (WHO) informal working group on echinococcosis. Guidelines for treatment of cystic and alveolar echinococcosis in humans. Bull WHO 1996;74:231-242.
- 33. Nilforoushzadeh MA, Hejazi SH, Zarkoob H, Shirani-Bidabadi L, Jaffary L. Efficacy of adding topical honey-based hydroalcoholic extract Nigella sativa 60% compared to honey alone in patients with cutaneous leishmaniasis receiving intralesional glucantime. J Skin Leishmaniasis 2010;1:1-7.
- 34. Fattahi Bafghi A, Vahidi AR, Anvari MH, Barzegar K, Ghafourzadeh M. The in vivo antileishmanial activity of alcoholic extract from Nigella sativa seeds. Afr J Microbiol Res 2011;5:1504-1510.
- 35. Okeola VO, Adaramoye OA, Nneji CM, Falade CO, Farombi EO, Ademowo OG. Antimalarial and antioxidant activities of methanolic extract of Nigella sativa seeds (black cumin) in mice infected with Plasmodium yoelli nigeriensis. Parasitol Res 2011;108:1507-1512.
- 36. Suthar MP, Patel PN, Shah TG, Patel RK. In vitro screening of Nigella sativa seeds for antifungal activity. Int J Pharm App Sci 2010;1:84-91.
- 37. El Daly ES. Protective effect of cysteine and vitamin E, Crocus sativus and Nigella sativa extracts on cisplatin-induced toxicity in rats. J Pharm Belg 1998;53:87-93.
- 38. Mansour MA. Protective effects of thymoquinone and desferrioxamine against hepatotoxicity of carbon tetrachloride in mice. Life Sci 2000;66:2583-2591.
- 39. Badary OA, Al-Shabanah OA, Nagi MN, Al-Bekairi AM, Elmazar MMA. Acute and subchronic toxicity of thymoquinone in mice. Drug Dev Res 1998;44:56-61.
Fig. 1Live protoscolices of Echinococcus granulosus after exposure to 0.1% eosin.
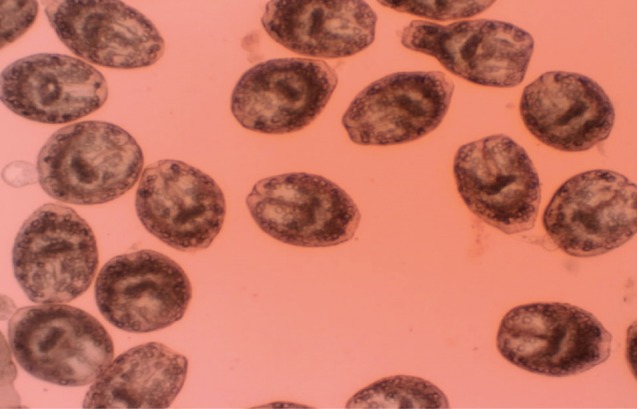
Fig. 2Dead protoscolices of Echinococcus granulosus after exposure to N. sativa and thymoquinone with 0.1% eosin.
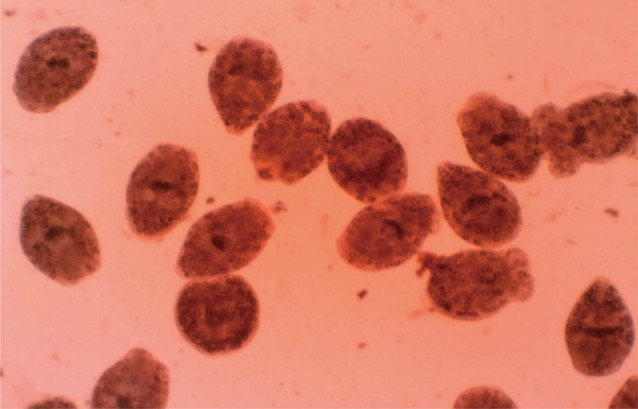
Table 1.Essential oil composition of N. sativa seeds identified by GC-MS
Table 1.
|
No |
Compound |
Percentage |
|
1 |
Camphene |
0.06 |
|
2 |
t-Anethole |
2.3 |
|
3 |
β-Pinene |
0.03 |
|
4 |
α-Pinene |
0.04 |
|
5 |
γ-Terpinene |
0.4 |
|
6 |
β-Myrcene |
0.05 |
|
7 |
α-Terpinene |
0.01 |
|
8 |
Limonene |
1.7 |
|
9 |
1,8-Cineole |
0.16 |
|
10 |
Sabinene |
1.3 |
|
11 |
ρ-Cymene |
14.1 |
|
12 |
α-Terpinolene |
0.01 |
|
13 |
ρ-Cymene-8-ol |
0.42 |
|
14 |
Carvacrol |
10.3 |
|
15 |
Longipinene |
0.4 |
|
16 |
Camphor |
1.5 |
|
17 |
Linaloolcis |
0.21 |
|
18 |
Sabinenehydrate |
0.2 |
|
19 |
Longifolene |
6.1 |
|
20 |
Bornylacetate |
0.53 |
|
21 |
Thymol |
1.2 |
|
22 |
4-Terpineol |
5.1 |
|
23 |
Borneol |
0.14 |
|
24 |
Carvone |
0.08 |
|
25 |
Thymoquinone |
42.4 |
|
26 |
2-Tridecanone |
0.8 |
|
27 |
Thujone |
1.5 |
|
28 |
ρ-Anisaldehyde |
0.1 |
|
29 |
2-Undecanone |
0.21 |
|
30 |
Unknown peak |
3.1 |
|
Total |
|
96.6 |
Table 2.Scolicidal effects of essential oil of N. sativa against protoscoleces of hydatid cyst at various concentrations following various exposure times
Table 2.
|
Concentration (mg/ml) |
Exposure time (min) |
Mean of mortality rate (%) |
|
10 |
5 |
82.3 |
|
10 |
100 |
|
20 |
100 |
|
30 |
100 |
|
60 |
100 |
|
5 |
21.3 |
|
1 |
10 |
67.6 |
|
20 |
100 |
|
30 |
100 |
|
60 |
100 |
|
5 |
8.3 |
|
0.1 |
10 |
21.6 |
|
20 |
32.3 |
|
30 |
55.6 |
|
60 |
76.6 |
|
5 |
4.6 |
|
0.01 |
10 |
14.6 |
|
20 |
23.3 |
|
30 |
36.3 |
|
60 |
52.6 |
|
5 |
0 |
|
Normal saline+Tween 20 |
10 |
1.3 |
|
20 |
2.6 |
|
30 |
4.3 |
|
60 |
6.6 |
|
20% hypertonic saline |
5 |
81.3 |
|
10 |
100 |
|
20 |
100 |
|
30 |
100 |
|
60 |
100 |
Table 3.Scolicidal effects of thymoquinone against protoscoleces of hydatid cyst at various concentrations following various exposure times
Table 3.
|
Concentration (mg/ml) |
Exposure time (min) |
Mean of mortality rate (%) |
|
1 |
5 |
58.6 |
|
10 |
100 |
|
20 |
100 |
|
30 |
100 |
|
60 |
100 |
|
5 |
18.3 |
|
0.5 |
10 |
38.6 |
|
20 |
100 |
|
30 |
100 |
|
60 |
100 |
|
5 |
7.3 |
|
0.25 |
10 |
17.6 |
|
20 |
35.6 |
|
30 |
96.3 |
|
60 |
100 |
|
5 |
3.3 |
|
0.125 |
10 |
19.6 |
|
20 |
21.3 |
|
30 |
39.6 |
|
60 |
87.3 |
|
5 |
0 |
|
Normal saline+Tween 20 |
10 |
1.3 |
|
20 |
2.6 |
|
30 |
4.3 |
|
60 |
6.6 |
|
20% hypertonic saline |
5 |
81.3 |
|
10 |
100 |
|
20 |
100 |
|
30 |
100 |
|
60 |
100 |