Abstract
Trichomonas vaginalis is a pathogen that triggers severe immune responses in hosts. T. vaginalis α-actinin 2, Tvα-actinin 2, has been used to diagnose trichomoniasis. This study was undertaken to examine the role of Tvα-actinin 2 as an antigenic molecule to induce immune responses from humans. Western blot analysis using anti-Tvα-actinin 2 antibodies indicated its presence in the secreted proteins of T. vaginalis. ELISA was employed to measure cytokine production by vaginal epithelial cells, prostate cells, mouse dendritic cells (DCs), or T cells stimulated with T. vaginalis or Tvα-actinin 2 protein. Both T. vaginalis and rTvα-actinin 2 induced cytokine production from epithelial cell lines, including IL-10. Moreover, CD4+CD25− regulatory T cells (Treg cells) incubated with rTvα-actinin 2-treated DCs produced high levels of IL-10. These data indicate that Tvα-actinin 2 modulates immune responses via IL-10 production by Treg cells.
-
Key words: Trichomonas vaginalis, α-actinin 2, Treg cell, interleukin-10 (IL-10)
INTRODUCTION
Trichomonas vaginalis, the causative pathogen for the most prevalent non-viral sexually transmitted disease in humans, triggers vaginitis, cervicitis, and urethritis [
1]. The impact of
T. vaginalis, as a model organism of host-parasite interaction has been extensively investigated, and multiple interactions between host receptors and protozoan virulence determinants are known to occur [
2,
3]. A previous study identified several antigenic proteins present in the membrane portion of
T. vaginalis, which includes α-actinin 2 [
4]. Alpha-actinin is a Ca
2+-regulated protein involved in actin cross-linking [
5]. Five putative α-actinin genes were discovered in the databases of
T. vaginalis. Among them,
T. vaginalis α-actinin 2 (Tvα-actinin 2) was investigated, due to its strong reactivity to patients’ sera [
6]. When the parasite adheres to the host cells and it transforms into the amoeboid form, this protein is located close to the cell surface showing colocalization with actin.
As the main antigen of
T. vaginalis, Tvα-actinin 2 has been used to monitor trichomoniasis in populations [
7] and to examine the history of trichomoniasis in plasma samples of male patients [
8]. Reactive epitopes of Tvα-actinin 2 have been finely mapped using sera from both male and female patients with trichomoniasis [
9]. In this study, Tvα-actinin 2 was found to be secreted from
T. vaginalis. We examined a possibility that this secreted protein of
T. vaginalis plays a role in the modulation of host immune responses using vaginal and prostate epithelial cells as well as mouse dendritic cells (DCs).
MATERIALS AND METHODS
Parasite cultivation
T. vaginalis KT4 [
10] was cultivated axenically in Diamond’s Trypticase Yeast extracted Maltose (TYM) medium [
11] with 10% horse serum (Gibco BRL, Karlsruhe, Germany). Cultures were grown at 37°C in a 5% CO
2, and transferred daily into fresh medium.
Bacterial and human cell culture
Escherichia coli BL21 (DE3) were grown at 37°C in Luria-Bertani medium (0.5% yeast extract, 1% tryptone, 1% NaCl, pH 7.5) supplemented with ampicillin (100 μg/ml) in order to maintain the plasmids. Medium components were obtained from BD-Difco (Franklin Lakes, New Jersey, USA), and the chemicals and antibiotics were purchased from Sigma-Aldrich (St. Louis, Missouri, USA).
Human vaginal epithelial cells (VECs, ATCC CRL-2614, American Type Culture Collection, Manassas, Virginia, USA) were grown in Dulbecco’s Modified Eagle’s Medium (Gibco BRL) complemented with 10% heat-inactivated fetal bovine serum (FBS), 100 U/ml penicillin, and 100 μg/ml streptomycin. Human prostatic epithelial cells (RWPE-1, ATCC CRL-11609, ATCC) were grown using Keratinocyte Serum Free Medium (Gibco BRL) containing 0.2 ng/ml epidermal growth factor and 0.05 mg/ml bovine pituitary extract, 5% FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin. These human epithelial cells were cultured to 80–90% confluence at 37°C/5% CO2.
Formation of Tvα-actinin 2-specific antibodies (Abs)
Full-length recombinant Tvα-actinin 2 (rTvα-actinin 2) was prepared as previously described [
12]. rTvα-actinin 2 was used as antigen to produce polyclonal Abs via intraperitoneal injection into pathogen-free rats (CrjBgi: CD[SD]IGS, 7-week-old, female). After 3 time injections at 2-week intervals, sera were obtained from immunized rats, and the titer of the resultant Abs was tested.
Secretomes were prepared from VECs, T. vaginalis trophozoites, and VECs incubated with T. vaginalis at a multiplicity of infection (MOI) of 5. VECs were seeded in 12-well culture plates (1×105 cells/well), and then incubated overnight at 5% CO2 and 37°C. T. vaginalis washed with phosphate buffered saline (PBS: 1.7 mM KCl, 137 mM NaCl, 2 mM KH2PO4, and 10 mM Na2HPO4, pH 7.3) were resuspended in the TYM media without serum. After 6 h-incubation of VEC with and T. vaginalis at a MOI of 5, the cells were removed by 20-min centrifugation at 1,200 g, and the collected secretomes were concentrated using a Centricone (Milipore, Darmstadt, Germany).
Ten micrograms of secreted proteins were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and transferred to a polyvinnylidene fluoride membrane (Millipore). The membrane was incubated with anti-rTvα-actinin 2 Abs (1:1,000 dilution) and subsequently with horseradish peroxidase (HRP)-conjugated mouse anti-rat IgG (1:1,000 dilution). Immunoreactive bands were visualized using an Enhanced Chemiluminescence (ECL) System (GE Healthcare, Chicago, Illinois, USA).
Determination of cytokine concentration
A sandwich ELISA was performed to determine the levels of TNF-α, IL-12, IL-6, and IL-10 in culture supernatants (BD Biosciences, Franklin Lakes, New Jersey, USA). The levels of cytokines were determined by monitoring the absorbance at 450 nm, and the cytokine concentrations were calculated based on the standard curves generated using recombinant cytokines.
To examine whether cytokine production in rTvα-actinin 2-induced VECs was caused by contamination with lipopolysaccharide (LPS) in the prepared rTvα-actinin 2, endotoxin level of rTvα-actinin 2 was measured using the Limulus Amebocyte Lysate LPS Detection Kit (Lonza, Basel, Switzerland). In addition, rTvα-actinin 2 were purified using the Detoxi-GelTM Endotoxin Removing Gel (Pierce, Rockford, Illinois, USA) before it was challenged to the host cells for measurement of cytokine production.
Experimental mice
BALB/c mice (6 week-old females) were purchased from OrientBio (Seongnam, Korea). Mice received care according to our institutional guidelines and the legal requirements of Korea.
Preparation of DCs
Mouse bone-marrow cells isolated from the tibia and femur of mice, were resuspended in red blood cell lysis buffer (10 mM KHCO3, 150 mM NH4Cl, and 1 mM EDTA, pH 7.4). The cells were differentiated into bone marrow-derived dendritic cells (BMDCs) in RPMI1640 medium (Gibco BRL) containing 1 mM HEPES buffer, 20 ng/ml granulocyte-macrophages colony-stimulating factor (Peprotech, Rocky Hill, New Jersey, USA), 100 U/ml penicillin/streptomycin (Gibco BRL), 10% FBS, 50 μM mercaptoethanol, and 0.1 mM nonessential amino acid (Sigma-Aldrich). The medium for these cells were replaced with the fresh one every 2 days, and the cultures were maintained at 37°C/5% CO2 until day 6 or 7.
Flow cytometric analysis of cell surface markers
Immature BMDCs were converted into mature cells by incubation with LPS for 16 hr. Mature DCs were harvested, and then reacted with rTvα-actinin 2 for additional 16 hr. The cells were then stained for 20 min on ice using the Live/dead Fixable Dead Cell Stain Kit (Invitrogen, Carlsbad, California, USA) to monitor their viability. Following 3 washes with PBS, the cells were reacted with one of these chemicals [allophycocyanin (APC)-conjugated anti-CD80, and phycoerythrin (PE)-conjugated anti-CD86, and peridinin chlorophyll (PerCP)-Cy 5.5-conjugated anti-mouse I-A/I-E for MHC class II] along with APC-eFluor780-conjugated anti-CD11c for 20 min. After 3 PBS-washes, the cells were prepared in 500 μl of PBS containing 1% BSA, and 0.1% sodium azide, and their fluorescence was measured by flow cytometry. The data analyses were performed using FlowJo data analysis software (FlowJo LLC, Ashland, Oregon, USA).
Assays of BMDC-dependent Treg differentiation
Naive T cells were obtained from the draining lymph nodes of mice. From the prepared cells in RBC buffer, CD4+CD25+ or CD4+CD25− regulatory T cells were prepared using CD4+ CD25+ Regulatory T cell Isolation Kit (Miltenyi Biotec, Auburn, California, USA) as directed by the manufacturer’s instructions. The isolated CD4+CD25+ or CD4+CD25− regulatory T cells were monitored for the expression of Tregs markers, FOXP3, CD103, CD39, and CD152 (CTLA-4) using specific Abs.
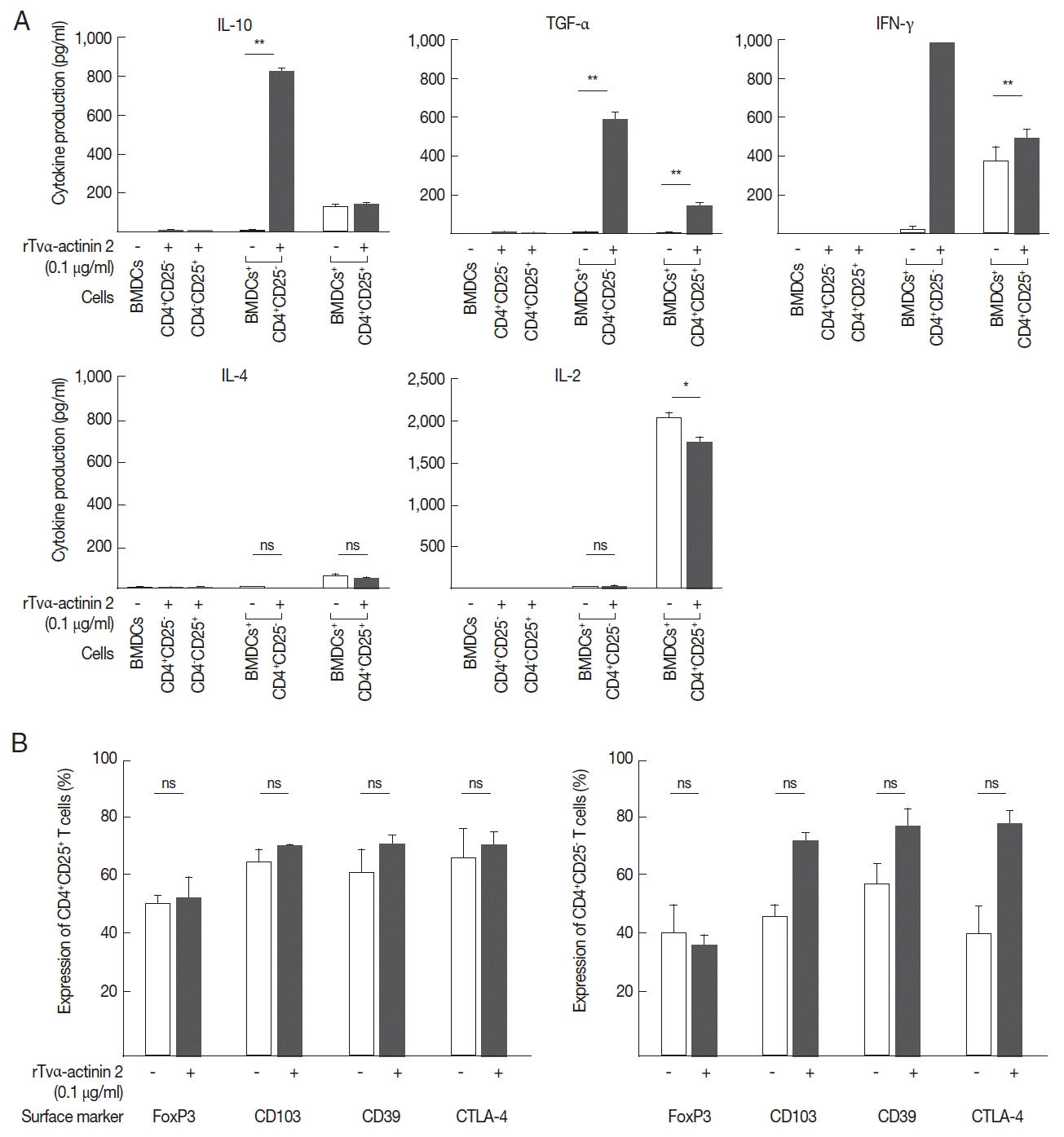
LPS-stimulated DCs (1×104 cells/well) incubated with 0.1 μg/ml of rTvα-actinin 2 for 3 h were co-cultured with CD4+ CD25+ or CD4+CD25− regulatory T cells (1×105) at a DC: T cell ratio of 1:10. On day 7 of co-culture, supernatants were collected and assayed for IL-10, TGF-α, IFN-γ, IL-4, and IL-2 production by ELISA (BD Biosciences). As a control, PBS-treated DCs was also assayed for these cytokines.
Statistical analyses
Data were indicated as the means±SD obtained from 3 independent experiments. Statistical analysis of pairwise comparison was carried out using Student’s t-test (SYSTAT program, SigmaPlot version 9, Systat Software Inc., San Jose, California, USA). Differences were considered statistically meaningful if the P-values were<0.05.
RESULTS
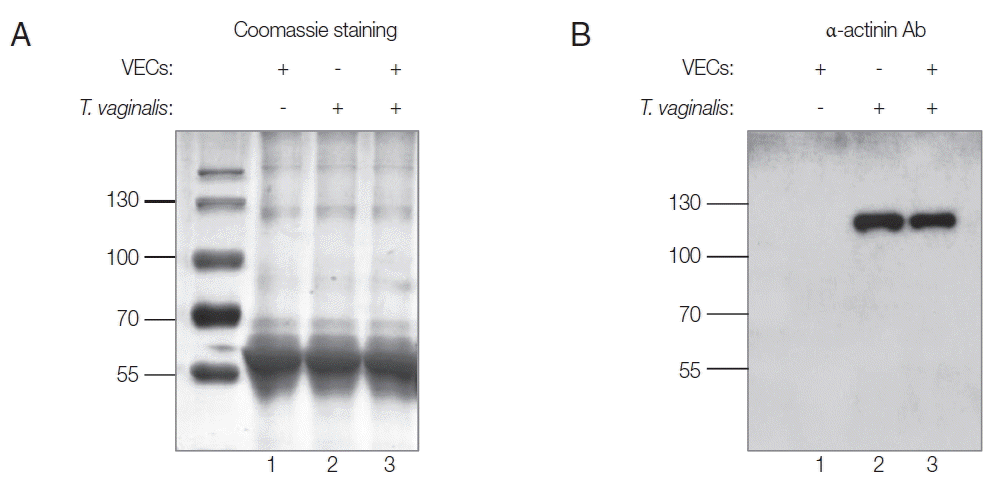
Secretion of Tvα-actinin 2 by T. vaginalis
In this study, rTvα-actinin 2 protein was used as an antigen to immunize rats to make anti-Tvα-actinin 2 antibodies. Using Abs specific to rTvα-actinin 2, western blot analysis was performed to examine the presence of Tvα-actinin 2 in secreted proteins by
T. vaginalis (
Fig. 1B). Secretomes were prepared from VECs (
Fig. 1A, B, lane 1) or
T. vaginalis (
Fig. 1A, B, lane 2). In addition, secreted proteins were also collected from
T. vaginalis incubated with VECs (
Fig. 1A, B, lane 3). No immunoreactive protein was observed in the western blot analysis of the secretome derived from VECs. In contrast, an immunoreactive protein of 124 kDa was clearly detected in the secretome from
T. vaginalis, which is an expected size of Tvα-actinin 2. The same reactive protein was observed in the secretomes of the VECs incubated with
T. vaginalis (
Fig. 1B). No obvious difference was detected in the amount of this immunoreactive protein between
T. vaginalis and
T. vaginalis incubated with VECs.
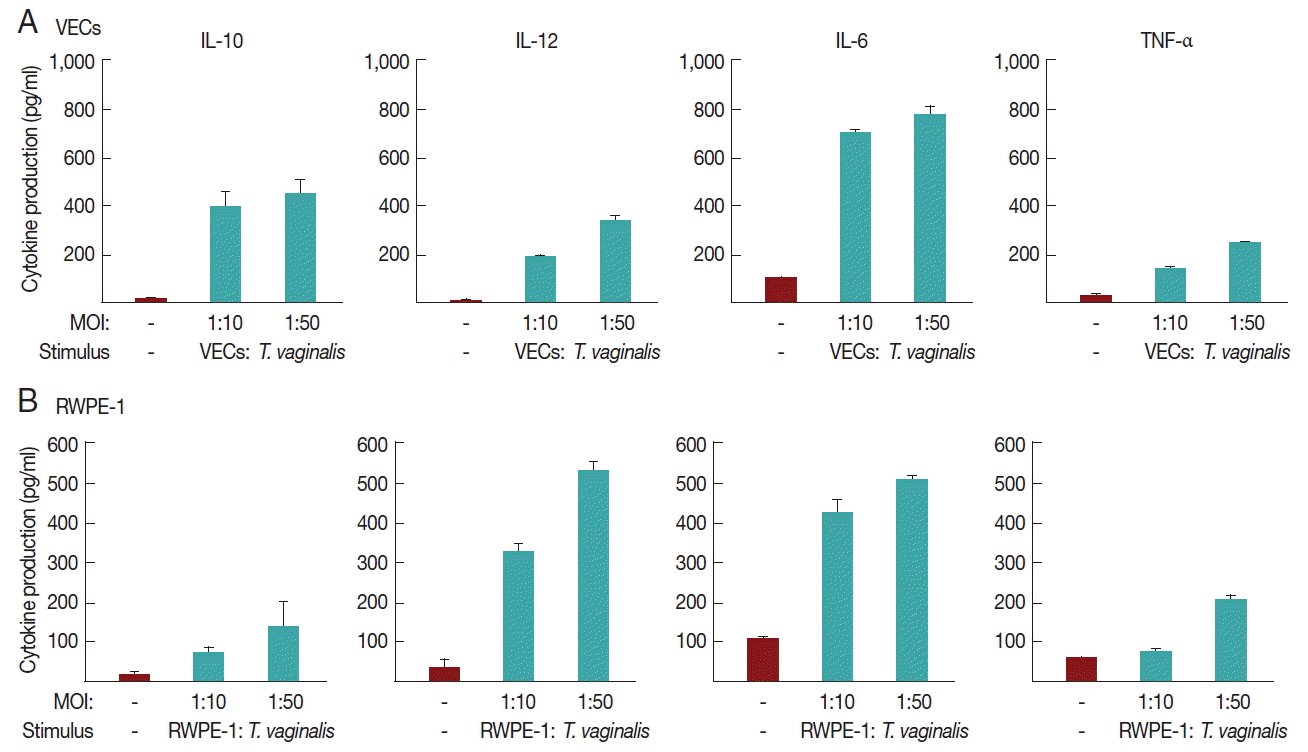
We investigated whether
T. vaginalis trophozoites induced cytokine production in VEC and RWPE-1, primary target cells that
T. vaginalis may encounter in vivo (
Fig. 2).
A 16-hr incubation of VECs with T. vaginalis at MOIs of 10 and 50 triggered the secretion of IL-10, IL-12, and IL-6, and TNF-α. The induction of IL-6 production by T. vaginalis occurred up to 770±39 pg/ml, whereas the amount of TNF-α was 250±2 pg/ml at an MOI of 50 of T. vaginalis. When VECs were stimulated with T. vaginalis at an MOI of 50, the levels of released IL-10 and IL-12 were 450±62 and 330±15 pg/ml, respectively. The levels of TNF-α and IL-12 were proportional to the MOI of T. vaginalis.
RWPE-1 cells were also stimulated with T. vaginalis at MOIs of 10, and 50, and then assayed for cytokine production. Similar levels of TNF-α, IL-10, IL-12, and IL-6 were released from RWPE-1 cells by T. vaginalis at levels of 140–510 pg/ml. The induced levels of these 4 cytokines were increased at a higher MOI of T. vaginalis.
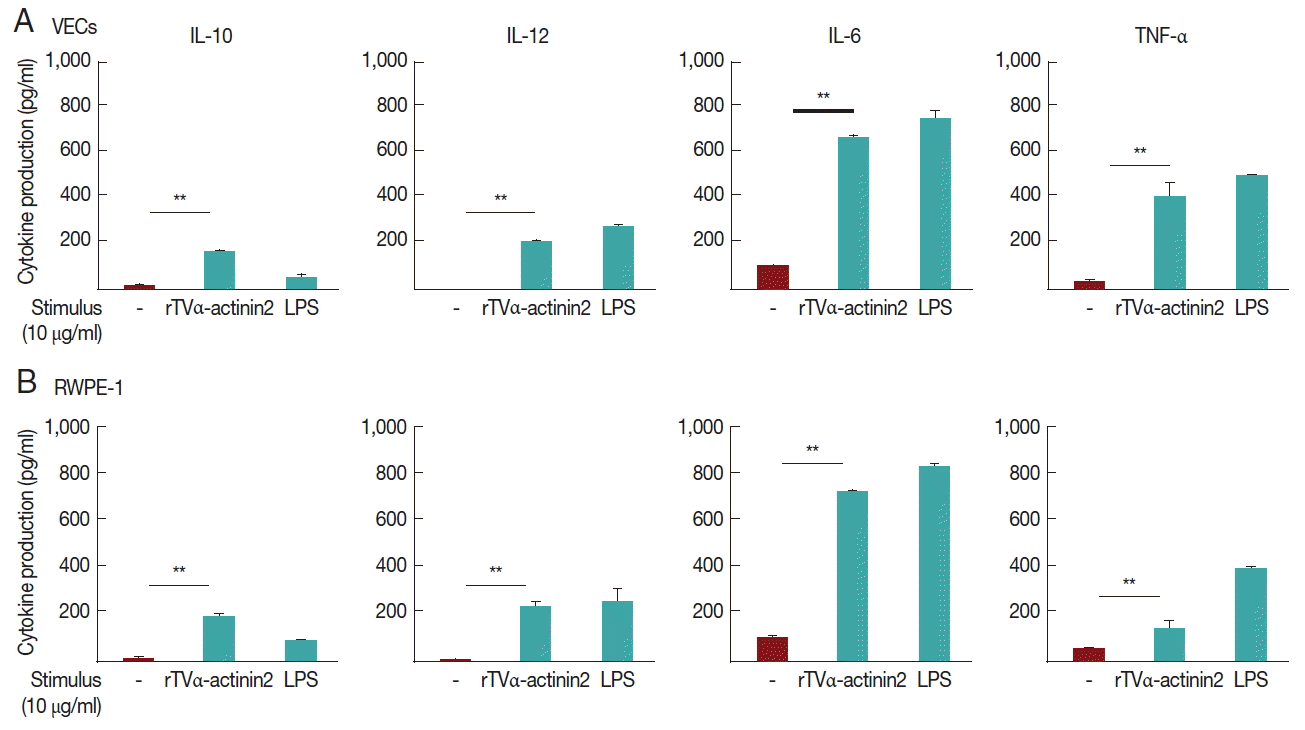
Cytokine production in VEC and RWPE-1 cells stimulated with rTvα-actinin 2
In this experiment, we examined whether rTvα-actinin 2 induces cytokine production in vaginal and prostate epithelial cell lines (
Fig. 3A, B, respectively). To exclude a possibility that contaminated LPS in purified rTvα-actinin 2 induced cytokine production, the recombinant protein was further purified using Endotoxin Removing Gel before it was challenged to the host cells. The endotoxin level of rTvα-actinin 2 was 0.8 EU/ml, which is lower than 2.5 EU/ml, the value indicating contamination.
When VECs were treated with rTvα-actinin 2 (10 μg/ml), all 4 cytokines (TNF-α, IL-10, IL-12, and IL-6) were released ranging from 160±8 to 730±35 pg/ml. Comparable amount of cytokines was produced in the rTvα-actinin 2-exposed RWPE-1 with VECs.
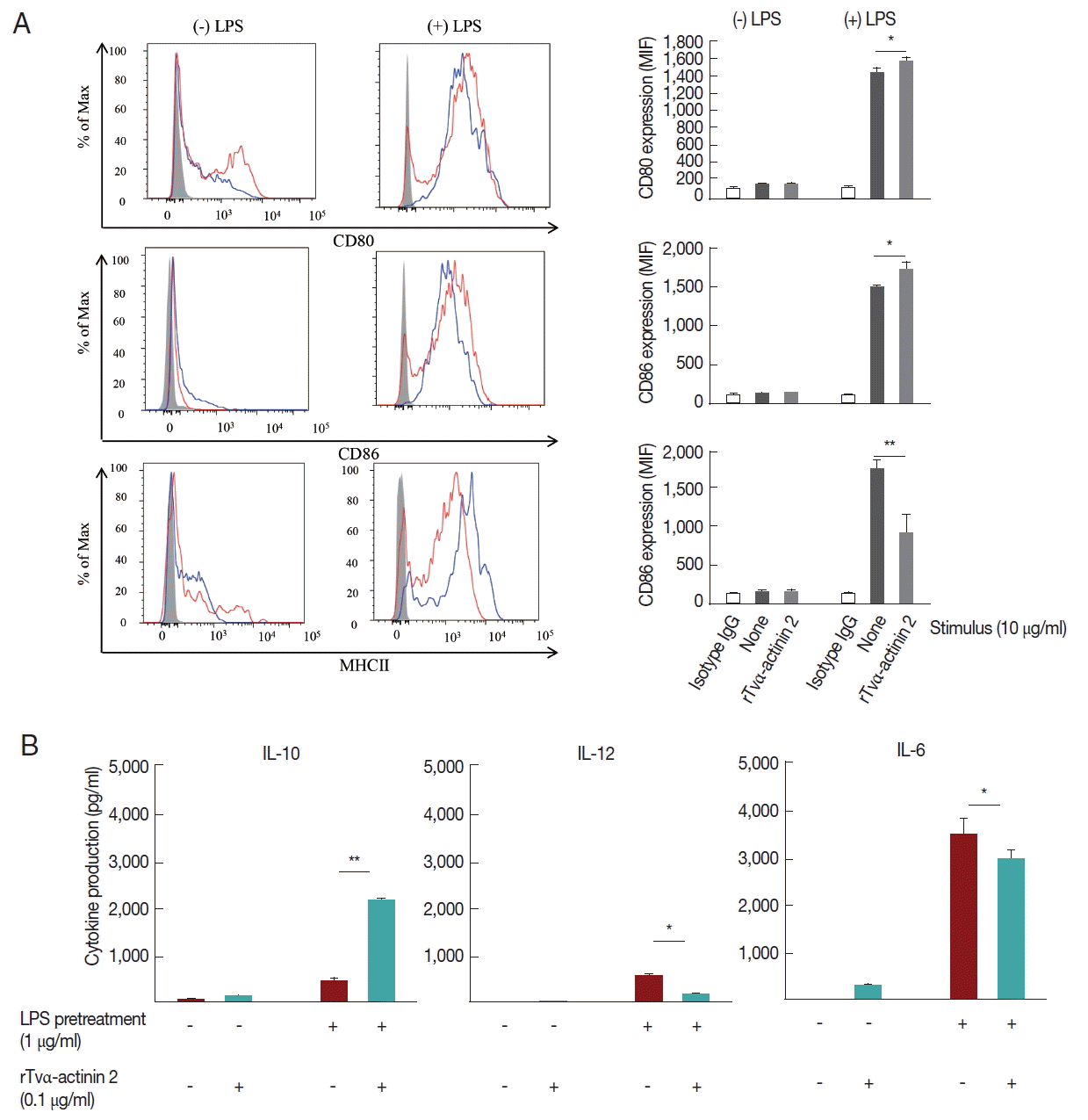
rTvα-actinin 2 induces a tolerogenic profile in DCs by down-regulating the surface molecule and increasing IL-10 production
Since the rTVα-actinin-treated host cells produced immunosuppressive cytokines as well as pro-inflammatory cytokines, we focused on the function of tolerogenic dendritic cells (tDCs) which can induce and control immune tolerance via differentiation of regulatory T cells (Tregs).
A set of mouse BMDCs was treated with LPS (1 μg/ml), and then incubated with 0.1 μg/ml rTvα-actinin 2 for 24 h. Another set of mouse BMDCs was treated with 0.1 μg/ml rTvα-actinin 2 for 24 h without LPS treatment. As a control, mouse BMDCs were treated with PBS instead of rTvα-actinin 2. The expression of DC activation markers, CD80, CD86, and MHC class II, was monitored in these cells using their specific antibodies (
Fig. 4A). Each set of BMDCs was incubated with isotype IgG as a control. All 3 DC activation markers showed increased expression in response to LPS treatment. The expression of CD80 and CD86 was not significantly altered by an additional treatment with rTvα-actinin 2. Interestingly, expression of MHC II decreased dramatically [from 1,762±11 to 915±250 Mean Fluorescence Intensity (MFI)] with an additional challenge of rTvα-actinin 2. This result indicated that
T. vaginalis α-actinin 2 down-regulates LPS-induced DC activation as shown by the decrease in MHC II expression.
Mouse BMDCs were stimulated with rTvα-actinin 2 (0.1 μg/ml), and then assayed for IL-10, IL-12, and IL-6 levels (
Fig. 4B). While the levels of IL-12 and IL-6 in LPS-challenged DCs were decreased by rTvα-actinin 2 treatment, IL-10 production was further increased by rTvα-actinin 2 in LPS-treated DCs. IL-10 plays an anti-inflammatory role to prevent inflammatory pathologies [
13].
In this experiment, we examined whether IL-10 is induced in regulatory T cells upon co-incubation with rTva-actinin 2-treated DCs. The regulatory T cells isolated from the spleen and draining lymph nodes of mice were further sub-fractionated into natural Tregs (nTregs; CD4
+CD25
+) and inducible Tregs (iTregs; CD4
+CD25
−). When the obtained CD4
+CD25
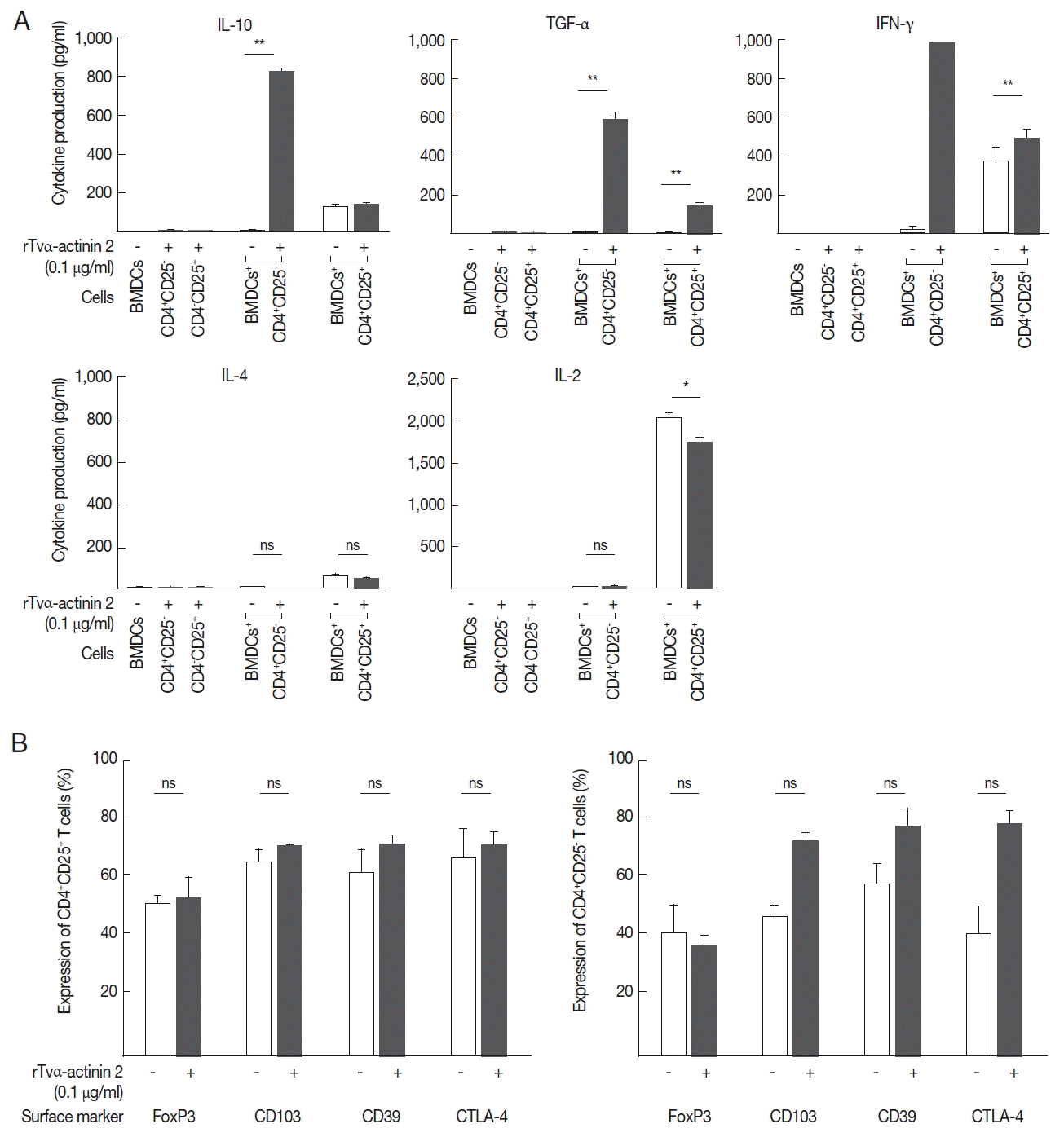
− T cells were co-incubated with LPS-stimulated mouse BMDCs treated with rTvα-actinin 2, they secreted a high level of IL-10 (825 pg/ml) compared to the cells incubated with LPS-stimulated DCs (9 pg/ml) (
Fig. 5A). In addition, these cells secreted a high level of TGF-β (585 pg/ml) and IFN-γ (1,000 pg/ml) only when they were co-incubated with LPS-stimulated DCs treated with rTvα-actinin 2. On the contrary, iTregs co-incubated with LPS-stimulated DCs treated with rTvα-actinin 2 did not release IL-4 or IL-2. On the other hand, nTregs secreted various amount of these cytokines ranging from 45 to 1,746 pg/ml when co-incubated with treated rTvα-actinin 2 of LPS-stimulated DCs. Except TGF-α, showing increased secretion after rTvα-actinin 2 treatment, nTregs demonstrated no alteration in cytokine levels in response to rTvα-actinin 2.
These Tregs were co-incubated with DCs treated only with LPS or DCs treated with LPS and rTvα-actinin 2, and the expression of FoxP3, CD103, CD39, and CTLA-4 was determined (
Fig. 5B). The expression of Treg markers such as FoxP3, CD103, CD39, and CTLA-4 was not significantly changed by an additional treatment of LPS-stimulated mouse BMDCs with rTvα-actinin 2 in the CD4
+CD25
+ cells. In case of CD4
+ CD25
− cells, the expression of 3 markers, CD103, CD39, and CTLA-4, was increased upon co-incubation with rTvα-actinin 2-treated DCs compared with that in cells co-incubated with the untreated DCs. On the contrary, FoxP3 level was not changed by rTvα-actinin 2 treated DCs. This result suggests that rTvα-actinin 2 induced the Treg phenotype in effector T lymphocytes.
DISCUSSION
Since Tvα-actinin 2 was identified as an immunogenic protein reacting to anti-
T. vaginalis IgGs of female patients with trichomoniasis [
7], it has even been used to monitor men’s serological samples for trichomoniasis exposure and risk of prostate cancer [
8]. Recently, this protein was finely mapped for epitopes reactive to patients’ sera from only females or from both males and females [
9]. To be functional with respect to host interaction, this protein in the pathogen should be secreted or be present on its outermost surface. In this study, Tvα-actinin 2 was found in the secretome of
T. vaginalis (
Fig. 1B) and its secretion was not affected by the presence of the host cells, VECs. Due to the lack of signal sequence in Tvα-actinin 2, it should be examined how Tvα-actinin 2 is secreted from
T. vaginalis. However, many proteins lacking signal sequences were found to be secreted via exocytosis [
14].
In this study, we examined immune responses of the host epithelial cells, especially vaginal and prostate cells by measuring cytokine production upon incubation with
T. vaginalis as well as rTvα-actinin 2 protein (
Figs. 2,
3, respectively). Among the produced cytokines, IL-10 attracted our attention because it is an anti-inflammatory cytokine preventing inflammatory pathologies [
15]. Therefore, we examined whether rTvα-actinin 2 plays a role in immunosuppression (
Fig. 4), clearly indicating that rTvα-actinin 2 modulates DC activation by down-regulation of IL-12/up-regulation of IL-10, and inhibition of LPS-induced maturation of DCs. Secretion of IL-10 by tDCs is essential for tolerance in Treg differentiation via several different pathways [
16].
Several components of
T. vaginalis modulate the host immune response. Human neutrophils and macrophages stimulated with
T. vaginalis produce IL-8 and the acute proinflammatory cytokine IL-6, TNF-α, and IL-1β, thereby initiate the immune response to
T. vaginalis infection [
10,
17]. Especially, IL-6 is a critical factor of chronic inflammation in prostate cancer [
18]. A recent study on interaction with prostate epithelial cells and
T. vaginalis demonstrated increased production of IL-6 and epithelial–mesenchymal transition markers [
19].
T. vaginalis lipophosphoglycan was shown to mediate adhesion of these parasites to VECs, and to induce a selective up-regulation of chemotactic cytokines in a TLR4-MyD88-independent manner [
20]. Screening of
T. vaginalis antigens with patients’ sera led to the isolation of
T. vaginalis enolase which has plasminogen binding activity and whose expression is up-regulated by contact between the parasite and VECs [
21]. Our results show immunosuppressive characteristics of the infection triggered by
T. vaginalis. Expression of IL-12 and TNF-α was suppressed in
T. vaginalis–adhesive cells, suggesting that this inhibitory mechanism is induced by
T. vaginalis to evade host immunity [
22]. Dying neutrophils by
T. vaginalis result in the resolution of vaginal inflammation by inducing anti-inflammatory cytokine production in human macrophages [
23]. In a study observing DC activation against various pathogens, formalin-fixed
T. vaginalis induces only IL-10, but not IL-12 [
24]. Importantly, excretory-secretory products (ESP) of
T. vaginalis triggered tolerance as shown the inhibition of LPS-induced maturation of DCs [
25]. Western blot analysis of
T. vaginalis ESP with anti-Tvα-actinin 2 revealed that this protein is also present in the secreted fraction (
Fig. 1B). Therefore, it is likely that Tvα-actinin 2 is one of the protozoan molecules functioning as an immunosuppressor during trichomoniasis, which acts both as a surface and secreted virulence factor. Interestingly,
T. vaginalis homolog of macrophage migration inhibitory factor, TvMIF was discovered to induce inflammation and cell proliferation, thus leading to the progression of prostate cancer [
26]. Identifying a host receptor for Tvα-actinin 2 would reveal the mechanisms underlying this protozoan molecule functions as a modulator for host immune systems.
Modulation of DC activity by rTvα-actinin 2 was mediated by Treg cells as shown in
Fig. 5. IL-10 is known to initiate an anti-inflammatory feedback loop by the cooperative action with other immune cells [
27]. The tDCs activated by IL-10 in peripheral tissues, obtain the ability to secrete IL-10, and then move to lymphoid organs, a site for Treg differentiation. We present evidence that Tvα-actinin 2 could generate iTregs during infection. Co-cultivation of Tvα-actinin 2 did not increase the levels of IL-10, TGF-β, and IFN-γ in nTregs (CD4
+CD25
+) but increased the levels of IL-10, TGF-β, and IFN-γ in CD4
+ CD25
− T cells. The high surface levels of CD103, CD39, and CTLA-4 on T effs (CD4
+CD25
−) help to contribute to tolerance of
T. vaginalis infections as a consequence of IL-10 production. Additionally, IL-10 is required for the suppressive phenotype of Tregs against the inflammatory signals. IFN-γ is a key factor for the conversion of CD4
+CD25
− T cells into iTreg as shown in suppressed autoimmunity of the IFN-γ deficient mice [
28].
Thus, our results demonstrated that rTvα-actinin 2 is responsible for immune tolerance, a characteristic of trichomoniasis by modulating the maturation and cytokine production by DCs.
Notes
-
CONFLICT OF INTEREST
We have no conflict of interest related to this work.
ACKNOWLEDGMENT
This work was supported by the Basic Science Research Programs (NRF-2016R1A6A3A11934451) through the National Research Foundation (NRF) of the Ministry of Education, Science, and Technology, Korea.
Fig. 1Secretomes from VECs (lane 1), T. vaginalis (lane 2), or VECs incubated with T. vaginalis (lane 3). Ten μg of secreted proteins was separated by 10% SDS-PAGE, and visualized by Coomassie staining (A). They were also incubated with anti-rTvα-actinin 2 Abs (1:1,000 dilution) and subsequently with HRP-conjugated anti-rat IgG (1:1,000 dilution). Immunoreactive bands were visualized using an ECL System (B).
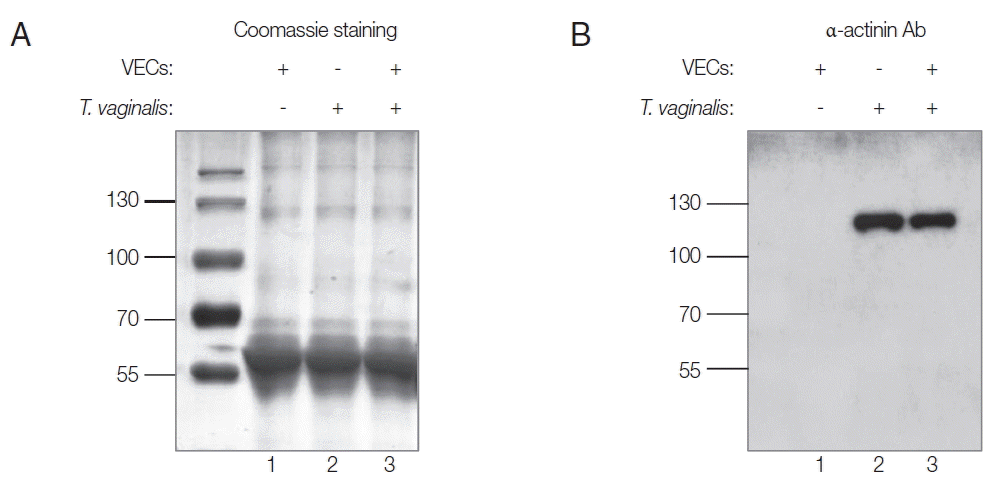
Fig. 2Cytokine production induced by T. vaginalis in VECs (A) and RWPE-1 (B). VEC or RWPE-1 cells (1×106 cells per well) were incubated in growth medium without antibiotics overnight and incubated with T. vaginalis trophozoites at MOIs of 10 or 50 for 16 hr. Cell-free supernatants were collected, and the levels of IL-10, IL-12, IL-6, and TNF-α were determined using ELISA kits.

Fig. 3Cytokine production induced by Tvα-actinin 2 in VECs (A) and RWPE-1 (B). VEC or RWPE-1 cells (1×106 cells per well) were incubated in growth medium without antibiotics overnight and incubated with 10 μg/ml rTvα-actinin 2 for 16 hr. As a positive control, the cells were treated with 10 μg/ml LPS for 16 hr. Cell-free supernatants were collected, and the levels of IL-10, IL-12, IL-6, and TNF-α were determined using ELISA kits. Values marked by asterisks are significantly different from the control (paired t-test, *P<0.05, **P<0.001).
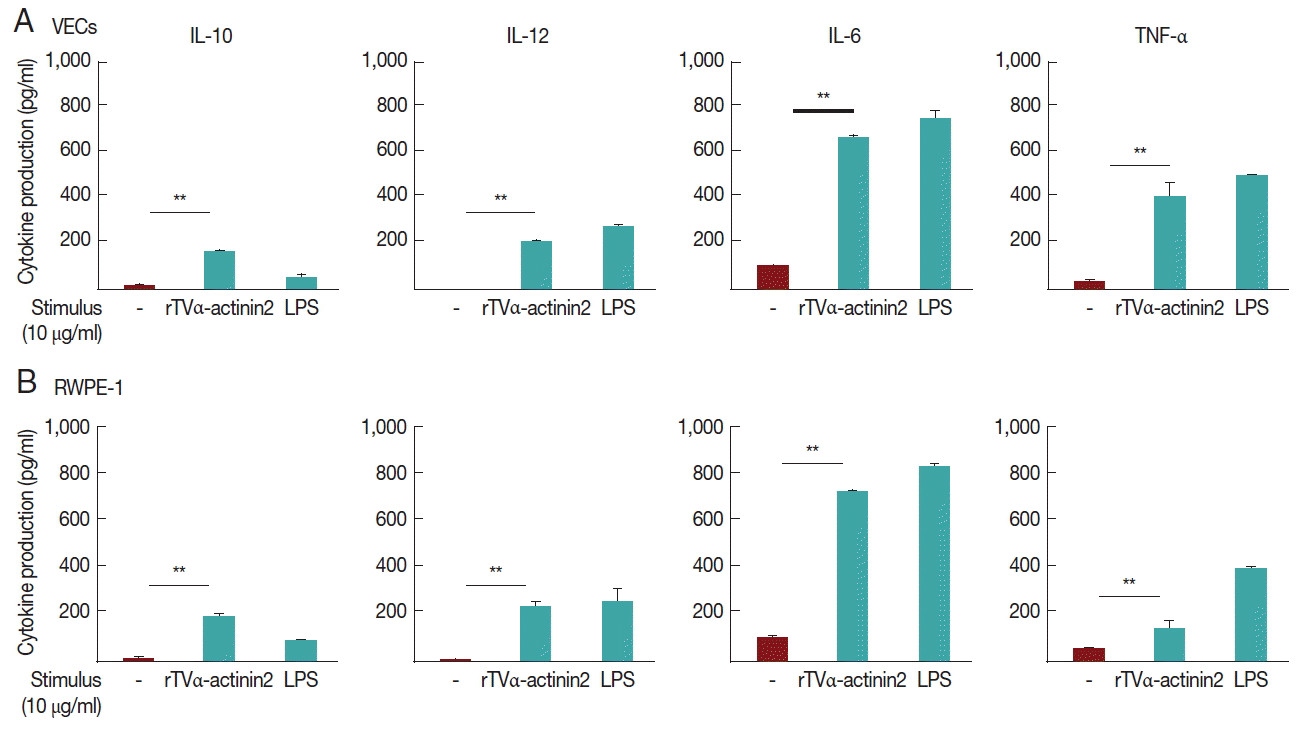
Fig. 4Cytokine production and expression of co-stimulatory and MHC II molecules by rTvα-actinin 2-stimulated mouse DCs. Mouse bone-marrow cells prepared from the femur and tibia of BALB/c mice were differentiated into DCs. On day 7, immature BMDCs (1×106 cells/ml) were stimulated with rTvα-actinin 2 (0.1 μg/ml) for 16 hr. Another set of cells were pre-treated with E. coli LPS (Sigma) at 0.5 μg/ml in addition to rTvα-actinin 2. (A) Cells were harvested and then stained with PerCP-Cy 5.5-conjugated anti-mouse I-A/I-E (MHC class II), APC-conjugated anti-CD80, and PE-conjugated anti-CD86 along with APC-eFlour780-conjugated anti-CD11c for 20 min on ice. Fluorescence was measured by flow cytometry, and data were analyzed using FlowJo data analysis software. Isotype controls are shown as shaded histograms, control BMDCs are shown as blue lines, and rTvα-actinin2 stimulated BMDCs are shown as red lines. Bar graphs shows surface markers as indicated as MFI of 3 independent experiments in which 3 mice were used to get BMDCs. (B) Cell-free supernatants were collected, and assayed for IL-10, IL-12, and IL-6 using ELISA kits. The negative control was supernatant of mouse immature DCs that were not stimulated. The assays were repeated 3 times. Each experiment was performed with a single mouse and the ELISA was performed in triplicate. A representative experiment is shown. Results are expressed as the mean±SD of the triplicate of the representative experiment. Statistical analyses for pair-wise comparisons were performed using Student’s t-tests to evaluate the statistical significance of these results. Differences with P-values of less than 0.05 were considered significant. Data with P-values of less than 0.001 are indicated with 2 asterisks.
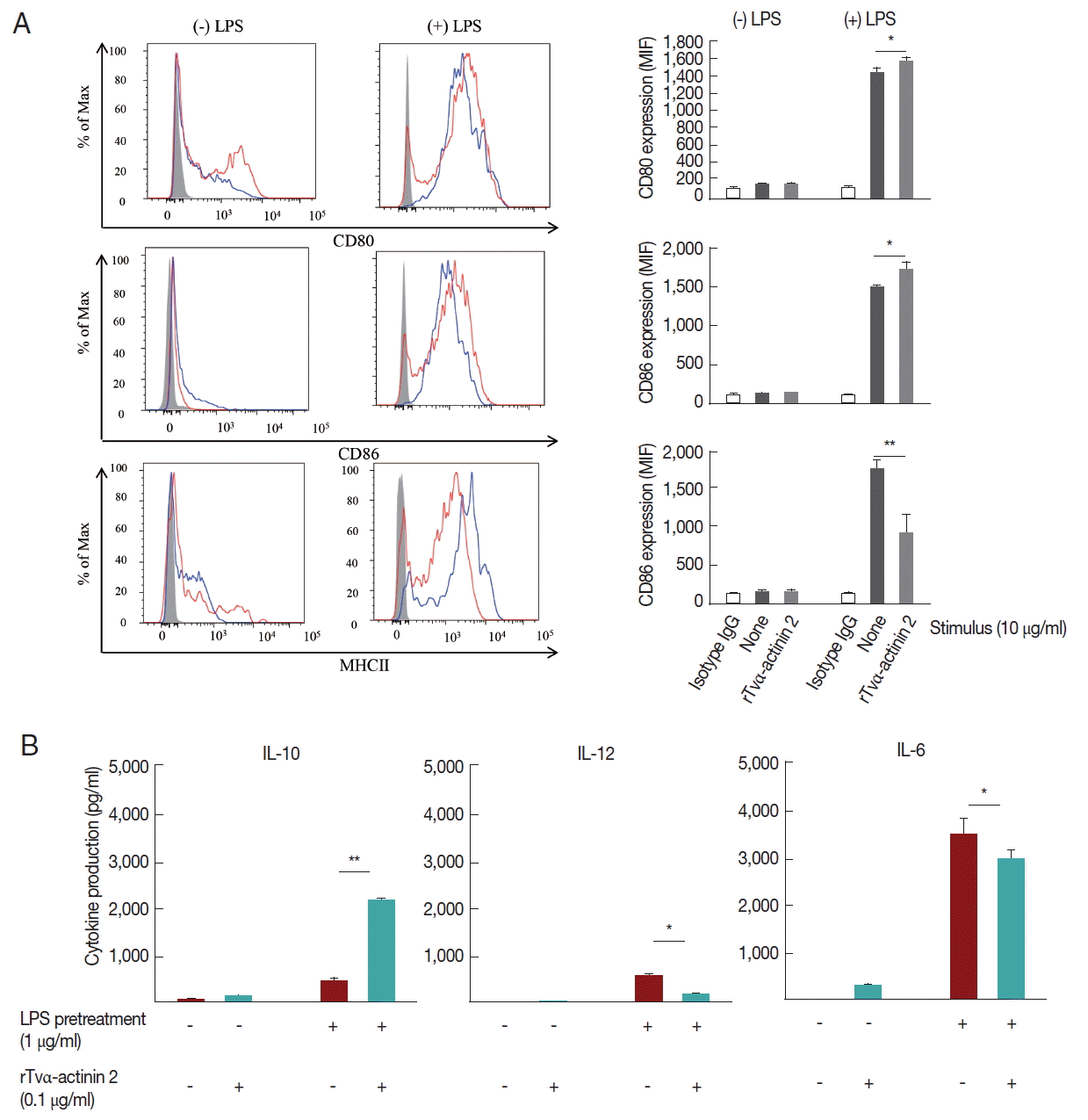
Fig. 5rTvα-actinin 2 induce the expression of a regulatory T cell profile in effector T cells. Mouse DCs treated with LPS were exposed to rTvα-actinin 2 and then incubated with mouse CD4+CD25+ or CD4+CD25− T cells. (A) Suppressive activity was determined by ELISA to measure IL-10, TGF-β, and IFN-γ secretion in the culture supernatants. Low/negative secretion was determined by IL-4 and IL-2 secretion in the culture supernatants. Figures are means of 3 independent experiments with 3 mice for each experiment. (B) CD4+CD25+ or CD4+CD25− lymphocytes were phenotyped by flow cytometry with PE-conjugated anti-CD25 and these populations were subsequently analyzed for expression of FoxP3, CD103, CD39, and CTLA-4. CD4+CD25+ or CD4+CD25− lymphocytes (1×105 cells/ml) were isolated from the spleen and draining lymph nodes of PBS or rTvα-actinin 2 incubated mouse BMDCs for 7 days co-incubation. Values marked by asterisks are significantly different from the control (paired t-test, n.s., not significant *P<0.05, **P<0.001).
References
- 1. Fouts AC, Kraus SJ. Trichomonas vaginalis: reevaluation of its clinical presentation and laboratory diagnosis. J Infect Dis 1993;141:137-143.
- 2. Fiori PL, Rappelli P, Addis MF. The flagellated parasite Trichomonas vaginalis: new insights into cytopathogenicity mechanisms. Microbes Infect 1999;1:149-156.
- 3. Figueroa-Angulo EE, Rendon-Gandarilla FJ, Puente-Rivera J, Calla-Chaoque JS, Cárdenas-Guerra RE, Ortega-López J, Quintas-Granados LI, Alvarez-Sánchez ME, Arroyo R. The effects of environmental factors on the virulence of Trichomonas vaginalis. Microbes Infect 2012;14:1411-1427.
- 4. Lee HY, Hyung S, Lee JW, Kim J, Shin MH, Ryu JS, Park SJ. Identification of antigenic proteins in Trichomonas vaginalis. Korean J Parasitol 2011;49:79-83.
- 5. Dixson JD, Forstner MJ, Garcia DM. The α-actinin gene family: a revised classification. J Mol Evol 2003;56:1-10.
- 6. Addis MF, Rappelli P, Delogu G, Carta F, Cappuccinelli P, Fiori PL. Cloning and molecular characterization of a cDNA clone coding for Trichomonas vaginalis alpha-actinin and intracellular localization of the protein. Infect Immun 1998;66:4924-4931.
- 7. Addis MF, Rappelli P, Pinto De Andrade AM, Rita FM, Colombo MM, Cappuccinelli P, Fiori PL. Identification of Trichomonas vaginalis alpha-actinin as the most common immunogen recognized by sera of women exposed to the parasite. J Infect Dis 1999;180:1727-1730.
- 8. Sutcliffe S, Giovannucci E, Alderete JF, Chang TH, Gaydos CA, Zenilman JM, De Marzo AM, Willett WC, Platz EA. Plasma antibodies against Trichomonas vaginalis and subsequent risk of prostate cancer. Cancer Epidemiol Biomarkers Prev 2006;15:939-945.
- 9. Neace CJ, Alderete JF. Epitopes of the highly immunogenic Trichomonas vaginalis α-actinin are serodiagnostic targets for both women and men. J Clin Microbiol 2013;51:2483-2490.
- 10. Ryu JS, Kang JH, Jung SY, Shin MH, Kim JM, Park H, Min DY. Production of interleukin-8 by human neutrophils stimulated with Trichomonas vaginalis. Infect Immun 2004;72:1326-1332.
- 11. Diamond LS. The establishment of various trichomonads of animals and man in axenic cultures. J Parasitol 1957;43:488-490.
- 12. Kim SR, Kim JH, Park SJ, Lee HY, Kim YS, Kim YM, Hong YC, Ryu JS. Comparison between mixed lysate antigen and α-actinin antigen in ELISA for serodiagnosis of trichomoniasis. Parasitol Int 2015;64:405-407.
- 13. Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol 1991;9:271-296.
- 14. Burgoyne RD. Secretory vesicle-associated proteins and their role in exocytosis. Annu Rev Physiol 1990;52:647-659.
- 15. Saraiva M, O’Garra A. The regulation of IL-10 production by immune cells. Nat Rev Immunol 2010;10:170-181.
- 16. Akbari O, Dekruyff RH, Umestsu DT. Pulmonary dendritic cells producing IL-10 mediate tolerance induced by respiratory exposure to antigen. Nat Immunol 2001;2:725-731.
- 17. Han IH, Goo SY, Park SJ, Hwang SJ, Kim YS, Yang MS, Ahn MH, Ryu JS. Proinflammatory cytokine and nitric oxide production by human macrophages stimulated with Trichomonas vaginalis. Korean J Parasitol 2009;47:205-212.
- 18. Sfanosn KS, De Marzo AM. Prostate cancer and inflammation: the evidence. Histopathology 2012;60:199-215.
- 19. Han IH, Kim JH, Kim SS, Ahn MH, Ryu JS. Signalling pathways associated with IL-6 production and epithelial-mesenchymal transition induction in prostate epithelial cells stimulated with Trichomonas vaginalis. Parasite Immunol 2016;38:678-687.
- 20. Fichorova RN, Trifonova RT, Gilbert RO, Costello CE, Hayes GR, Lucas JJ, Singh BN. Trichomonas vaginalis lipophosphoglycan triggers a selective upregulation of cytokines by human female reproductive tract epithelial cells. Infect Immun 2006;74:5773-5779.
- 21. Mundodi V, Kucknoor AS, Alderete JF. Immunogenic and plasminogen-binding surface-associated α-enolase of Trichomonas vaginalis. Infect Immun 2008;76:523-531.
- 22. Chang JH, Ryang YS, Morio T, Lee SK, Chang EJ. Trichomonas vaginalis inhibits proinflammatory cytokine production in macrophages by suppressing NF-κB activation. Mol Cells 2004;18:177-185.
- 23. Ahn MH, Song HO, Ryu JS. Trichomonas vaginalis-induced neutrophil apoptosis cause anti-inflammatory cytokine production by human monocyte-derived marcophages. Parasite Immunol 2008;30:410-416.
- 24. Scott K, Manunta M, Germain C, Smith P, Jones M, Mitchell P, Dessi D, Branigan Bamford K, Lechler RI, Fiori PL, Foster GR, Lombardi G. Qualitatively distinct patterns of cytokines are released by human dendritic cells in response to different pathogens. Immunology 2005;116:245-254.
- 25. Song MJ, Lee JJ, Nam YH, Kim TG, Chung YW, Kim M, Choi YE, Shin MH, Kim HP. Modulation of dendritic cell function by Trichomonas vaginalis-derived secretory products. BMB Rep 2015;48:103-108.
- 26. Twu O, Dessí D, Vu A, Mercer F, Stevens GC, de Miguel N, Rappelli P, Cocco AR, Clubb RT, Fiori PL, Johnson PJ. Trichomonas vaginalis homolog of macrophage migration inhibitory factor induces prostate cell growth, invasiveness, and inflammatory responses. Proc Natl Acad Sci U S A 2014;111:8179-8184.
- 27. Murai M, Turovkaya O, Kim G, Madan R, Karp CL, Cheroutre H, Kronenberg M. Interleukin 10 acts on regulatory T cells to maintain expression of the transcription factor Foxp3 and suppressive function in mice with colitis. Nat Immunol 2009;10:1178-1184.
- 28. Wang Z, Hong J, Sun W, Xu G, Li N, Chen X, Liu A, Xu L, Sun B, Zhang JZ. Role of IFN-gamma in induction of Foxp3 and conversion of CD4+CD25− T cells to CD4+ Tregs. J Clin Invest 2006;116:2434-2341.