Abstract
Chigger mites are parasites of rodents and other vertebrates, invertebrates, and other arthropods, and are the only vectors of scrub typhus, in addition to other zoonoses. Therefore, investigating their distribution, diversity, and seasonal abundance is important for public health. Rodent surveillance was conducted at 6 districts in Shandong Province, northern China (114–112°E, 34–38°N), from January to December 2011. Overall, 225/286 (78.7%) rodents captured were infested with chigger mites. A total of 451 chigger mites were identified as belonging to 5 most commonly collected species and 3 genera in 1 family. Leptotrombidium scutellare and Leptotrombidium intermedia were the most commonly collected chigger mites. L. scutellare (66.2%, 36.7%, and 49.0%) was the most frequently collected chigger mite from Apodemus agrarius, Rattus norvegicus, and Microtus fortis, respectively, whereas L. intermedia (61.5% and 63.2%) was the most frequently collected chigger mite from Cricetulus triton and Mus musculus, respectively. This study demonstrated a relatively high prevalence of chigger mites that varied seasonally in Shandong Province, China.
-
Key words: Leptotrombidium scutellare, Leptotrombidium intermedia, chigger mite, species, rodent, Shandong Province, China
INTRODUCTION
Chigger mites belonging to the families Leeuwenhoekiidae and Trombiculidae (suborder Actinetidida, order Trombidiformes, subclass Acari, and class Arachnida) are ectoparasitic mites, which can lead to skin disorders, e.g., trombidiosis (trombiculiasis). Moreover, chigger mites are commonly regarded as vectors of scrub typhus, a disease caused by the intracellular pathogen
Orientia tsutsugamushi [
1–
5]. Worldwide, a total of 3,000 chigger mite species that parasitize mammals (especially rodents), birds, reptiles, amphibians, invertebrates, and other arthropods have been recorded, with > 420 species reported in China [
6–
8].
O. tsutsugamushi, a gramnegative bacterium, is transmitted transovarially to larval chigger mites that infest and transmit the bacterium to various hosts, including humans [
9,
10]. Scrub typhus has been reported in southern China (south of the Yangtze River) for decades, with the main epidemic season occurring during the summer months. Scrub typhus was not previously reported in northern China (north of the Yangtze River), including Shandong Province, until 1986, but is now considered to be an emerging infectious disease in this area, with seasonal epidemics occuring during the autumn and winter seasons [
11,
12]. The annual incidence of scrub typhus increased from 0.23 to 0.64 per 100,000 people from 2006 to 2012 in Shandong Province, China [
13]. Thus, studies of rodent populations and their associated ectoparasites provide information on the seasonal and geographic distributions of chigger mites. This study reports on the species composition, species diversity, and seasonal distributions of chigger mites in Shandong Province, China.
MATERIALS AND METHODS
Ectoparasite collection and identification
Rodents were collected from 6 districts (Binzhou, Liaocheng, Heze, Jining, Linyi, and Qingdao) in Shandong Province, north China (114–112°E, 34–38°N) from January to December 2011 (
Fig. 1). Rodent live capture traps (7.5× 9× 23 cm; HC Hareware Products Co., Ltd, Tianjin, China) were set at 17:00–18:30 hr and retrieved early the following morning (07:30–08:30 hr), and were set in both indoor (every 15 m
2 at rodent haunts) and outdoor habitats bordering rivers, canals, roads, bridges, burial sites, and residential areas. Traps with rodents were placed in white cloth bags labelled with the time, date and location, and then placed in a styrofoam cooler and transported to our laboratory where they were euthanized in accordance with an approved animal use protocol and then identified to species based on their general external morphological features [
14,
15].
Chigger mites were collected from rodent hosts using curettes and lancets, and then placed in individually labelled vials containing 70% ethanol [
16]. All mites were rinsed with distilled water to remove the ethanol and then mounted on glass slides with Hoyer’s medium, and later identified at 400× microscope (Olympus CX41, Hamburg, Germany) [
15,
17]. Voucher chigger mites and representative rodents were deposited at the Medical Entomology Department, Shandong Institute of Parasitic Diseases, China.
The proportion (
P) of each chigger mite species, the chigger mite infestation rate (
Ir), and the mean number of chigger mites per rodent host (
MA) were calculated [
18,
19], where
P=NiN×100%;Ir=HpHT×100%; and
MA=MHT×100% (
Ni = the number of each specific mite species,
N= the total number chiggers from all mite species,
HP = the number of infested rodents,
HT = the total number of rodents, and
M= the mean number of each species of mites. The above indices were calculated using SPSS, version 16.0 (SPSS, Chicago, Illinois, USA).
RESULTS
Chigger mite species
A total of 225/286 (78.7%) rodents collected from the 6 survey locations were infested with chigger mites. A total of 451 chigger mites were identified as belonging to the 5 most commonly collected species and 3 genera 1 family. Leptotrombidium scutellare and Leptotrombidium intermedia were the 2
L. scutellare (66.2%, 36.7%, and 49.0%) was the most frequently collected chigger mite from
Apodemus agrarius, Rattus norvegicus, and
Microtus fortis, respectively, while
L. intermedia (61.5% and 63.2%) was the most frequently collected from
Cricetulus triton and
Mus musculus, respectively (
Table 1) .
Infestation rate of chigger mites (Ir)
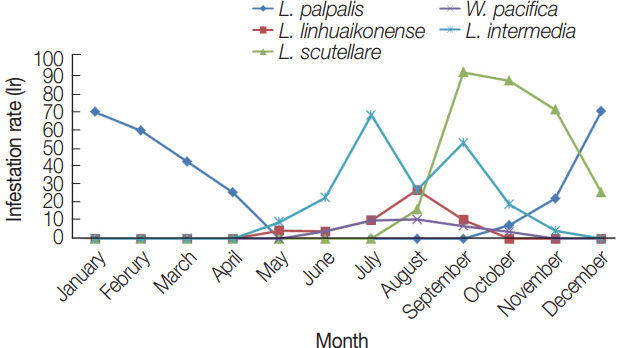
L. palpalis was collected from January to April and from October to December 2011 (
Fig. 2). The percent of rodents infested decreased from high in December (68.8%) to low in May–September and then increased from October (7.4%) to highs in December (68.8%) and January (68.4%) the following year.
Leptotrombidium linhuaienense was collected from May to September, with the highest infestation indices occurring from July to September (10.0–26.3%).
Walchia pacifica was collected from June to October with infestation indices increasing slowly and peaking in August (10.5%).
L. scutellare was collected from August to December with infestation indices of 89.7%, 85.2%, and 69.6% for September, October, and November, respectively, and was the most frequently collected mite species during the autumn season.
L. intermedia mites were collected from May to November with infestation indices ranging from 26.3% to 66.7%, and were more frequently collected from July to September.
Mean no. of chigger mites per rodent host (MA)
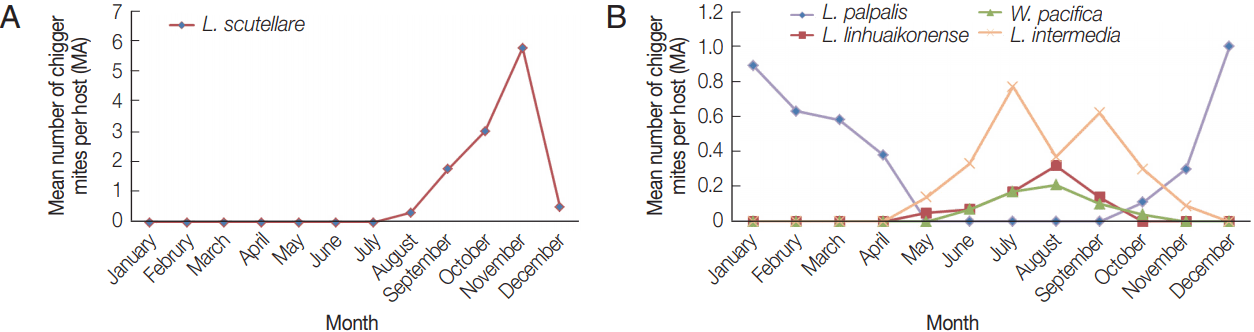
L. scutellare was the most commonly collected chigger mite with mean numbers increasing from July (0.32), reaching 1.76, 3.00, and 5.74 in September, October, and November, respectively (
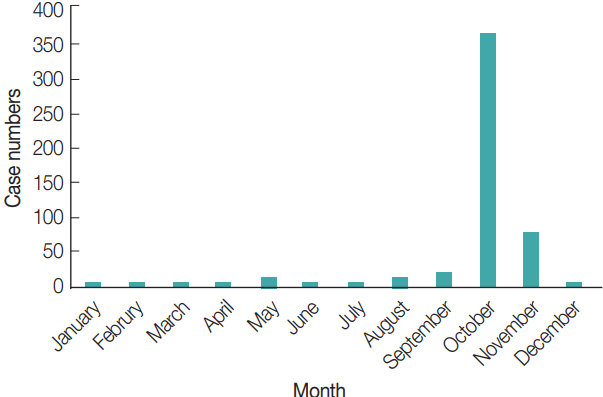
Fig. 3A). The reported cases of scrub typhus in September, October, and November were 13, 357, and 70, respectively (
Fig. 4). Thus, the seasonal distribution of scrub typhus corresponded with the seasonal fluctuations in the prevalence of
L. scutellare. The MA for
L. palpalis decreased gradually from January (0.89) to April (0.38) and increased again from October (0.11) to December (1.00) (
Fig. 3B). The MA for
L. linhuaienense increased from May (0.05) to August (0.32), while the MA for
W. pacifica increased gradually from June (0.07) to August (0.21), and then decreased in September (0.10) and October (0.04). The MA for
L. intermedia increased gradually from May (0.14) to July (0.77), and then decreased gradually from October (0.30) to November (0.09).
DISCUSSION
Scrub typhus, transmitted by larval chigger mites, is endemic in the Asia-Pacific region [
20–
23]. A total of 10,860 chigger mites collected from small mammals in South Korea were identified as only 8 species belonging to 4 genera [
4], and a survey in Japan identified a total of 16,369 individual chigger mites belonging to only 10 species and 3 genera [
3]. In contrast, the species diversity of chigger mites in China is much higher than the diversity cited in reports on chigger mites in other Asian countries, with > 400 chigger mite species that vary among the different provinces. For example, 18, 12, 15, and 41 chigger mite species were collected from small mammals (
A. agrarius, R. norvegicus, M. musculus, C.triton, Rattus flavipectus, and
Apodemus speciosus) from Liaoning, Jilin, Heilongjiang, and Hubei Provinces, respectively [
24]. To date, at least 17 chigger mite species, belonging to 6 genera and 3 families have been identified from Shandong Province [
25,
26]. The present investigation resulted in the identification of the 5 most commonly collected chigger mite species belonging to 3 genera in 1 family.
L. scutellare was the predominant chigger mite species collected, and
A. agrarius was the primary host for all the chigger species, a finding consistent with previous studies [
24–
26]. Our study showed a low species dominance, which may imply low thresholds for specific host preferences, and may increase their probability of encountering humans, as well as their transmission of scrub typhus among various hosts [
27,
28]. Chigger mite species composition has a comparatively high species richness and diversity, which may be attributed to the ecological situation in Shandong Province [
29–
31], which has a temperate and monsoonal climate suitable for various rodent hosts and chigger mite survival.
Seasonal differences in the relative abundance of chigger mites showed that L. palpalis infestations occurred mainly during autumn and winter, and that it was the dominant species collected during the winter. L. linhuaienense, W. pacifica, and L. intermedia were collected more frequently during the summer and were the dominant species collected during that period. L. scutellare appeared in the autumn and early winter and was the dominant species collected from September to November and corresponded to higher numbers of scrub typhus in humans, with the majority of patients reporting to health clinics beginning in late August, numbers peaking in October, and then declining to low numbers in December. Shandong Province is an economically developed province in China, with new rural reconstruction and urbanization, and invasion of rodent habitats, there is increased opportunity for mites to come into contact with the humans.
This study has provided key scientific data that identifies potential disease risks for the development of preventive and potential control measures against scrub typhus in Shandong Province. However, future studies should aim to increase the rodent host sample size and diversity and investigate in greater detail the overall geographical distribution of chigger mites in Shandong Province.
Notes
-
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
ACKNOWLEDGMENTS
We are grateful to staff members Huai-Wei Wang and Hai-Fang Wang for their contributions in previous field collection and investigations and participating student Qi-Qi Shi at Shandong Institute of Parasitic Diseases. This project was financed by grants from the National Natural Science Foundation of China (grant nos. 81672059 and 81471985), Natural Science Foundation of Shandong province (grant nos. ZR2015YL032, ZR2014YL031, and ZR2015YL023), the Scientific Research Foundation of Jining Medical University (grant no. JY2015BS26), and the Innovation Project of Shandong Academy of Medical Sciences.
Fig. 1Six small mammal and chigger mite collection sites in Shandong Province, northern China.
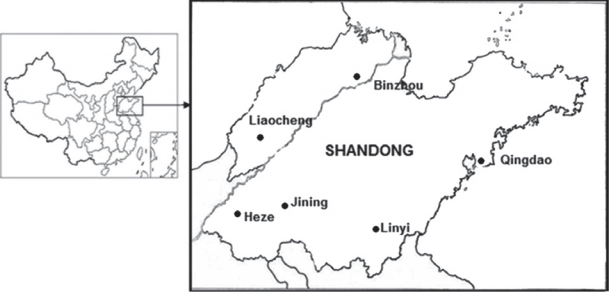
Fig. 2Seasonal variations of the infestation rate (Ir) in Shandong Province, northern China.
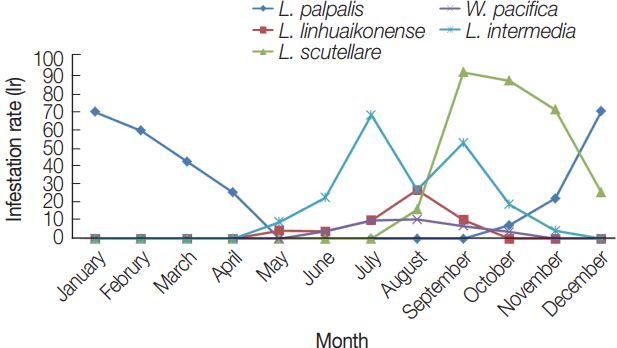
Fig. 3(A) Seasonal variations of the mean number of Leptotrombidium scutellare per rodent host (MA) in Shandong Province, northern China. (B) Seasonal variations of the mean number of Leptotrombidium palpalis, Leptotrombidium linhuaienense, Walchia pacifica, and Leptotrombidium intermedia per rodent host (MA) in Shandong Province, northern China. most commonly collected chigger mite species.
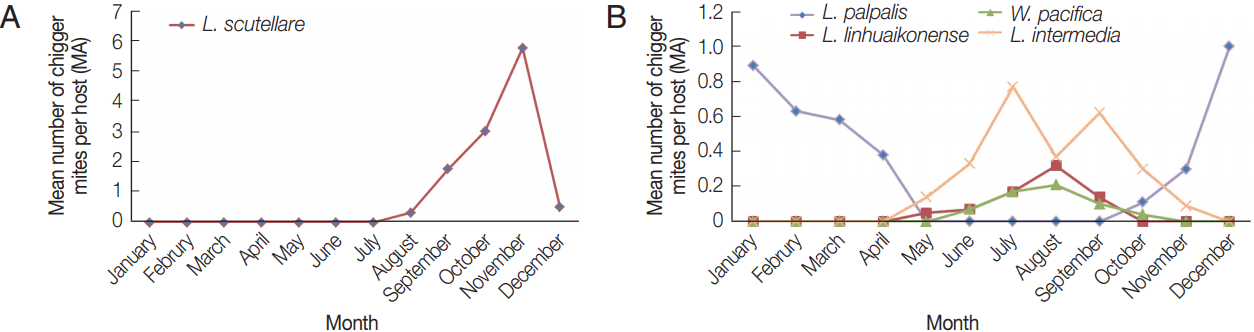
Fig. 4Seasonal distribution of scrub typhus cases in Shandong Province, China.
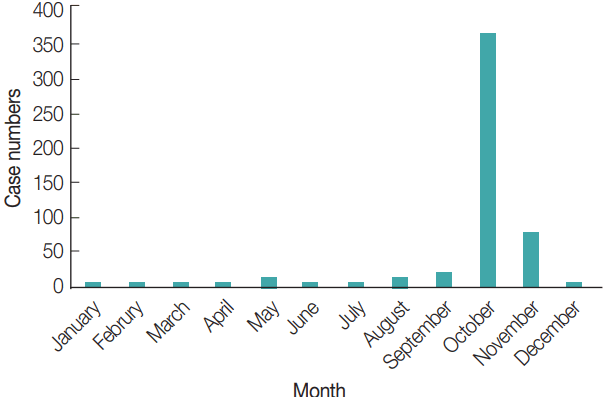
Table 1No. of rodents collected, no. (%) infested with the 5 most commonly collected chigger mites, and the mean no. of chigger mites per rodent host
Table 1
|
Rodent Species |
No. Rodent collected |
No. (%)Rodent infested |
L. scutellare
|
L. palpalis
|
L.linhuaikonense
|
W. pacifica
|
L. intermedia
|
Total |
Chigger mite indexa
|
|
|
|
|
|
|
No. (%)collected |
Chigger mite indexa
|
No. (%)collected |
Chigger mite indexa
|
No. (%)collected |
Chigger mite indexa
|
No. (%)collected |
Chigger mite indexa
|
No. (%)collected |
Chigger mite indexa
|
|
A. agrarius
|
167 |
136 (81.4) |
190 (66.2) |
1.40 |
53 (18.5) |
0.39 |
14 (4.9) |
0.10 |
10 (3.5) |
0.07 |
20 (7.0) |
0.15 |
287 |
2.11 |
|
|
R. norvegicus
|
36 |
22 (61.1) |
11 (36.7) |
0.50 |
9 (30.0) |
0.41 |
0 (0.0) |
0.00 |
0 (0.0) |
0.00 |
10 (33.3) |
0.45 |
30 |
1.36 |
|
|
C. triton
|
15 |
12 (80.0) |
0 (0.0) |
0.00 |
0 (0.0) |
0.00 |
0 (0.0) |
0.00 |
5 (38.5) |
0.42 |
8 (61.5) |
0.67 |
13 |
1.08 |
|
|
M. musculus
|
7 |
7 (100.0) |
7 (36.8) |
1.00 |
0 (0.0) |
0.00 |
0 (0.0) |
0.00 |
0 (0.0) |
0.00 |
12 (63.2) |
1.71 |
19 |
2.71 |
|
|
M. fortis
|
61 |
48 (78.7) |
50 (49.0) |
1.04 |
21 (20.6) |
0.44 |
4 (3.9) |
0.08 |
0 (0.0) |
0.00 |
27 (26.5) |
0.56 |
102 |
2.13 |
|
|
Total |
286 |
225 (78.7) |
258 (57.2) |
1.15 |
83 (18.4) |
0.37 |
18 (0.0) |
0.08 |
15 (0.0) |
0.07 |
77 (17.1) |
0.34 |
451 |
2.00 |
References
- 1. Varma RN. Prevalence of Leptotrombidium deliense, the scrub typhus vector, in the eastern Himalayas. Nature 1969;222:984-985.
- 2. Asanuma K, Kitaoka M, Shimizu F, Kano R.
Leptotrombidium scutellare as a vector of scrub typhus at the endemic area of the foothills of Mt. Fuju, Japan. J Hyg Epidemiol Microbiol Immunol 1974;18:172-184.
- 3. Iwasa M, Kasuya S, Noda N, Hioki A, Ito A, Ohtomo H. Trombiculid mites (Acari: Trombiculidae) and Rickettsia tsutsugamushi isolated from wild rodents in a new endemic area of Japan. J Med Entomol 1990;27:501-508.
- 4. Lee IY, Kim HC, Lee YS, Seo JH, Lim JW, Yong TS, Klein TA, Lee WJ. Geographical distribution and relative abundance of vectors of scrub typhus in the Republic of Korea. Korean J Parasitol 2009;47:381-386.
- 5. Yu J, Deng XZ, Yang ZQ, Yao PP, Zhu HP, Xiong HR, Li CL, Zhang Y. Study on the transmission of Hantaan virus and Orientia tsutsugamushi by naturally dual infected Leptotrombidium scutellare through biting. Zhonghua Yu Fang Yi Xue Za Zhi 2010;44:324-328. (in Chinese).
- 6. Daniel M, Stekolnikov AA. Chigger mites (Acari: Trombiculidae) new to the fauna of Cuba, with the description of two new species. Folia Parasitol (Praha) 2003;50:143-150.
- 7. Daniel M, Stekolnikov AA. Chigger mites (Acari: Trombiculidae) from Makalu region in Nepal Himalaya, with a description of three new species. J Med Entomol 2009;46:753-765.
- 8. Loan HK, Cuong NV, Takhampunya R, Klangthong K, Osikowicz L, Kiet BT, Campbell J, Bryant J, Promstaporn S, Kosoy M, Hoang NV, Morand S, Chaval Y, Hien VB, Carrique-Mas J.
Bartonella species and trombiculid mites of rats from the Mekong Delta of Vietnam. Vector Borne Zoonotic Dis 2015;15:40-47.
- 9. Jensenius M, Fournier PE, Raoult D. Rickettsioses and the international traveler. Clin Infect Dis 2004;39:1493-1499.
- 10. Cho NH, Kim HR, Lee JH, Kim SY, Kim J, Cha S, Kim SY, Darby AC, Fuxelius HH, Yin J, Kim JH, Kim J, Lee SJ, Koh YS, Jang WJ, Park KH, Andersson SG, Choi MS, Kim IS. The Orientia tsutsugamushi genome reveals massive proliferation of conjugative type IV secretion system and host-cell interaction genes. Proc Natl Acad Sci U S A 2007;104:7981-7986.
- 11. Yang LP, Zhao ZT, Liu YX, Feng YQ, Wang XJ, Li Z. Genotype identification and sequence analysis of Orientia tsutsugamushi isolated from Shandong area. Zhonghua Liu Xing Bing Xue Za Zhi 2006;27:1061-1064. (in Chinese).
- 12. Zhang M, Zhao ZT, Wang XJ, Li Z, Ding L, Ding SJ. Scrub typhus: surveillance, clinical profile and diagnostic issues in Shandong, China. Am J Trop Med Hyg 2012;87:1099-1104.
- 13. Wu YC, Qian Q, Magalhaes RJ, Han ZH, Haque U, Weppelmann TA, Hu WB, Liu YX, Sun YS, Zhang WY, Li SL. Rapid increase in scrub typhus incidence in Mainland China, 2006–2014. Am J Trop Med Hyg 2016;94:532-536.
- 14. Deng GF, Wang DQ, Gu YM, Meng YC. Economic insect fauna of China. Fasc 40, Acari, Dermanyssoidea. Science Press; Beijing, China. 1993, pp 62-320.
- 15. Huang WJ, Chen YX, Wu D, Zhou DH. Rodents of China Fudan. University Press; Shanghai, China. 1995, pp 1-308.
- 16. Durden LA, Ellis BA, Banks CW, Crowe JD, Oliver JH Jr. Ectoparasites of gray squirrels in two different habitats and screening of selected ectoparasites for Bartonellae
. J Parasitol 2004;90:485-489.
- 17. Li JC, Wang DQ, Chen XB. Trombiculid mites of China. Guangdong Science and Technology Press; Guangzhou, China. 1997, pp 97-438.
- 18. Bush AO, Lafferty KD, Lotz JM, Shostak AW. Parasitology meets ecology on its own terms: Margolis et al. revisited. J Parasitol 1997;83:575-583.
- 19. Men XY, Guo XG, Dong WG, Niu AQ, Qian TJ, Wu D. Ectoparasites of Chevrier’s field mouse, Apodemus chevrieri, in a focus of plague in southwest China. Med Vet Entomol 2007;21:297-300.
- 20. Kweon SS, Choi JS, Lim HS, Kim JR, Kim KY, Ryu SY, Yoo HS, Park O. Rapid increase of scrub typhus, South Korea, 2001–2006. Emerg Infect Dis 2009;15:1127-1129.
- 21. Wu YC, Qian Q, Magalhaes RJ, Han ZH, Haque U, Weppelmann TA, Wang Y, Liu YX, Li XL, Sun HL, Sun YS, Clements AC, Li SL, Zhang WY. Spatiotemporal dynamics of scrub typhus transmission in mainland China, 2006–2014. PLoS Negl Trop Dis 2016;10:e0004875.
- 22. Lee HW, Cho PY, Moon SU, Na BK, Kang YJ, Sohn Y, Youn SK, Hong Y, Kim TS. Current situation of scrub typhus in South Korea from 2001–2013. Parasit Vectors 2015;8:238.
- 23. Takahashi M, Misumi H, Urakami H, Nakajima S, Furui S, Yamamoto S, Furuya Y, Misumi M, Matsumoto I. Mite vectors (Acari: Trombiculidae) of scrub typhus in a new endemic area in northern Kyoto, Japan. J Med Entomol 2004;41:107-114.
- 24. Liu Y, Zhao Z, Yang Z, Zhang J, Xu J, Wu Q, Peng Z, Miao Z. Epidemiological studies on host animals of scrub typhus of the autumn-winter type in Shandong Province, China. Southeast Asian J Trop Med Public Health 2003;34:826-830.
- 25. Xue J, Zhou GZ, Liu YX. The faunal study of chigger mites in Shandong Province. Zhongguo Meijie Shengwuxue Ji Kongzhi Za Zhi 2004;15:452-454. (in Chinese).
- 26. Yang Z, Liu Y, Yu X, Wu Q, Xing R. Investigation on natural foci of autumn-winter type tsutsugamushi disease in Shandong Province. Zhonghua Liu Xing Bing Xue Za Zhi 2000;21:283-286. (in Chinese).
- 27. Poulin R, Krasnov BR, Shenbrot GI, Mouillot DK, Hokhlova IS. Evolution of host specificity in fleas: is it directional and irreversible. Int J Parasitol 2006;36:185-191.
- 28. Zhang L, Zhao Z, Bi Z, Kou Z, Zhang M, Yang L, Zheng L. Risk factors associated with severe scrub typhus in Shandong, northern China. Int J Infect Dis 2014;29:203-207.
- 29. Krasnov BR, Shenbrot GI, Khokhlova IS, Degen AA. Flea species richness and parameters of host body, host geography and host ‘milieu’. J Animal Ecol 2004;73:1121-1128.
- 30. Yang LP, Liu J, Wang XJ, Ma W, Jia CX, Jiang BF. Effects of meteorological factors on scrub typhus in a temperate region of China. Epidemiol Infect 2014;142:2217-2226.
- 31. Ding L, Wang XJ, Li Z, Ding SJ, Zhang M, Zhao ZT. Epidemic characteristics and related factors of autumn-winter type scrub typhus in Shandong area, 2010. Chin J Public Health 2013;29:543-545. (in Chinese).