Abstract
Trichostrongylus eggs observed in cellophane-thick smears are difficult, in practice, to distinguish from hookworm eggs. In order to overcome these limitations, a molecular approach was conducted. A Trichostrongylus colubriformis adult worm was obtained from a human in Laos, which was identified morphologically. ITS-1 sequence of this worm was determined, and found to be most similar with that of T. colubriformis among the Trichostrongylus spp. reported so far. Then, this sequence was compared with those of human hookworm species, Ancylostoma duodenale and Necator americanus, and species-specific oligonucleotide primers were designed. Polymerase chain reaction (PCR) using these primers evidenced specifically amplified PCR products of Trichostrongylus sp., A. duodenale and N. americanus from the eggs of each (520 bp, 690 bp, and 870 bp, respectively). A species-specific PCR technique can be developed in order to study the epidemiology of Trichostrongylus spp. and hookworms in endemic areas.
-
Key words: Trichostrongylus colubriformis, Ancylostoma duodenale, Necator americanus, PCR diagnosis, ITS-1
The genus
Trichostrongylus are primarily parasites of herbivores, and are distributed throughout the world. Human infections associated with several
Trichostrongylus spp. have been occasionally reported from many regions, and are accidental.
Trichostrongylus orientalis has been isolated fairly frequently from humans in Korea, Japan, China, and Armenia (
John and Petri, 2006). Human infections with
T. orientalis,
T. colubriformis,
T. vitrinus,
T. axei,
T. capricola,
T. probolurus, and
T. skrjabini have been previously reported in Iran, of which
T. orientalis and
T. colubriformis were more frequently detected than others (
Ghadirian and Arfaa, 1975). Trichostrongyles are related to hookworms in terms of transmission mode, morphology, and pathophysiology. Hookworms are of significant importance to human health, and the identification of different hookworm species is also quite important for the study of epidemiology, population biology, and anthelminthic efficacy, all of which are factors essential to hookworm control.
Adult worms of
Trichostrongylus spp. are much smaller than hookworms, but their eggs are larger. Currently, there is a paucity of available information regarding the epidemiology and population biology of
Trichostrongylus spp. This lack of information is due in part to the difficulty inherent to the differentiation of
Trichostrongylus from the 2 most important human hookworm species,
Ancylostoma duodenale and
Necator americanus, at certain life-cycle stages via the comparison of morphological features. The differentiation of eggs from those of hookworms can be accomplished when eggs are clearly observed via microscopy after the application of the formalin-ether concentration technique. However, it is difficult to identify various
Trichostrongylus species from the eggs and to distinguish the eggs from those of the hookworm, especially on cellophane-thick smears. Although the diagnosis of
Trichostrongylus infection is essential for the control of parasitic infections, current methods for the detection of this parasite from feces are normally predicated on the observations of light microscopy. These methods are time-consuming, and it remains difficult to distinguish
Trichostrongylus eggs from other eggs that are morphologically similar to
Trichostrongylus spp. Verweij et al. (
2000) employed the polymerase chain reaction (PCR) method utilizing genetic markers in ribosomal DNA for the specific amplification of minute amounts of
Oesophagostomum bifurcum eggs from northern Togo, where the human hookworm,
N. americanus, also exists with a high prevalence and has eggs that are morphologically similar to the latter. The assay achieved a sensitivity of 94.6% and 100% specificity. Similarly, we attempted to distinguish between hookworms and
Trichostrongylus spp. via PCR in this study.
Stool examinations were conducted nationwide in Laos for the purposes of intestinal parasite control in schoolchildren, between 2000 and 2005 (
Rim et al., 2003). The overall hookworm infection rate was 19.1%, although this figure may have included
Trichostrongylus infections. The eggs of these 2 species were so morphologically similar that it was difficult, in practice, to distinguish between hookworm and
Trichostrongylus eggs on a cellophane-thick smear. However, we identified a large number of
Trichostrongylus eggs from Laotian stool specimens which were taken to a laboratory in Korea and examined, which appeared larger in size and had more pointed ends than those of hookworms via microscopy, after the application of the formalin-ether concentration method (data not shown). In addition to extensive stool examination and mass anti-helminthic treatment, intestinal parasitic worms were acquired on a small scale for the purposes of this study. Adult worms of
N. americanus,
A. duodenale, and
Trichostrongylus spp. were collected from several infected people, after treatment with 10 mg/kg of pyrantel pamonate (Combantrin
®, Pfizer, New York, USA), purgation with magnesium sulfate (30 g) and water, in Vientiane Municipality (November, 2002) in Khamouane Province (March, 2003) and Saravane Province (November, 2003), Laos.
Morphological examinations revealed characteristics of hookworms and
Trichostrongylus colubriformis, respectively (
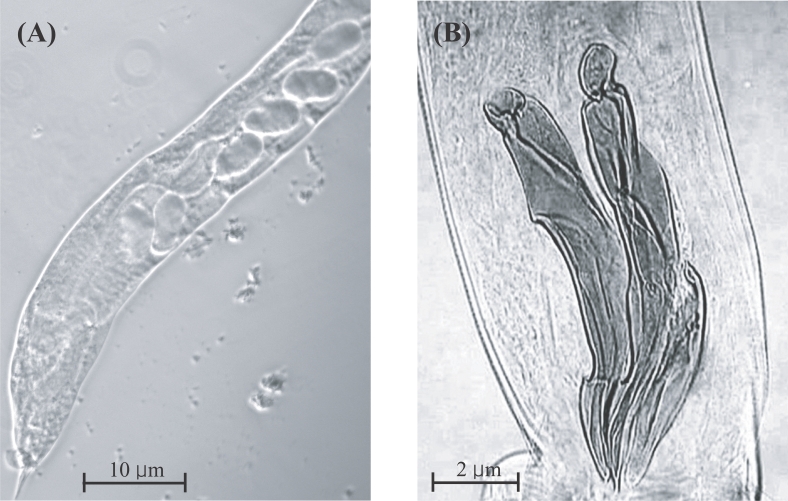
Beaver et al., 1984). Uterine eggs from a female and a pair of spicules of a male
T. colubriformis are shown in
Fig. 1. This is the first report of a
T. colubriformis adult worm infection in Laotians. Previously
Trichostrongylus infections were diagnosed by stool examinations only without definite species identification. The worms were fixed and stored in 70% ethanol until the isolation of DNA for genetic studies.
The isolation of genomic DNA from adult worms and the cloning of the ITS region were conducted. The genetic region was selected as per the suggestions of previous workers who reported that it might be useful to provide phylogenetic information at higher taxonomic levels within the superfamily Ancylostomatoidea (
Chilton and Gasser, 1999). Genomic DNA was isolated from adult worms via sodium dodecylsulphate/proteinase K treatment, and purified using Wizard
™ DNA Clean-UP columns (Promega, Madison, Wisconsin, USA). The rDNA region encompassing the ITS-1, 5.8S, ITS-2 plus approximately 50 nucleotides of the 28S rRNA gene was PCR amplified using the NC5 and NC2 primers (
Gasser et al., 1996;
Monti et al., 1998;
Chilton and Gasser, 1999). Amplifications were conducted in 50 µl volumes using 5-50 ng DNA, 12.5 pmol of each primer, 1 unit of Taq DNA polymerase, 250 µM of each dNTP, 10% glycerol, 0.01% gelatin, and 1 mM MgCl
2. The sample mixtures were heated for 5 min to 94℃ followed by 30 cycles of 94℃ for 1 min, 52℃ for 40 sec, and 72℃ for 90 sec, followed by 15-min cycle at 72℃. The PCR products were cloned into pGEM-T Easy vector (Promega) for DNA sequencing. The ITS-1 region was then sequenced to both orientations of the
Trichostrongylus NC5-NC2 PCR product, using the universal sequencing primers T3 and T7. ITS-1 sequence of a
Trichostrongylus sp. was determined. This sequence is most similar to that of
T. colubriformis among the reported
Trichostrongylus spp. Only 2 out of 390 bases were different from
T. colubriformis ITS-1 sequence in the GenBank. The nucleotide sequence of ITS regions of
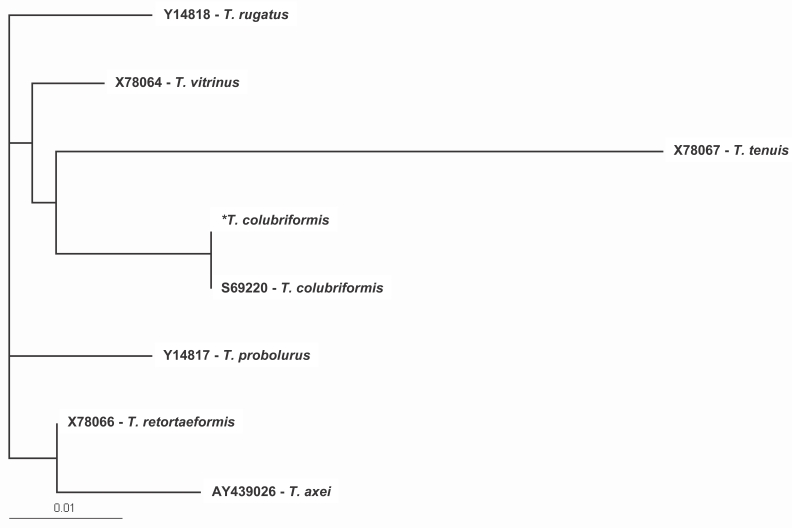
Trichostrongylus spp. were analyzed by multiple alignment analysis using the Clustal X multiple alignment program, and phylogenetic analysis was performed using the PAUP
* (version 4.0) program (
Fig. 2). There is, however, no
T. orientalis sequence thus far available in the database.
Then, this sequence was compared with those of the hookworms,
A. duodenale and
N. americanus. The nucleotide sequences of ITS-1 were aligned using the Clustal X Program (
Thompson et al., 1997) (
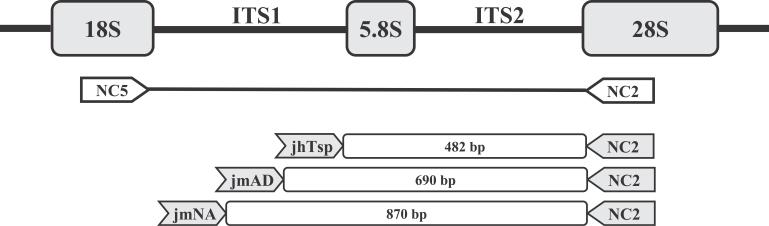
Fig. 3). Specific oligonucleotide primers were targeted toward the regions of difference in the DNA sequences between the species (
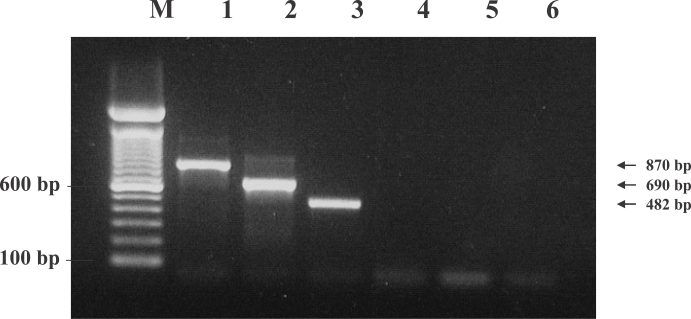
Fig. 4). Using these primers, a PCR technique was developed for the specific amplification of the DNA of the
Trichostrongylus sp.,
A. duodenale and
N. americanus, which generated PCR products of 482 bp, 690 bp, and 870 bp, respectively, on 2% agarose gel electrophoresis (
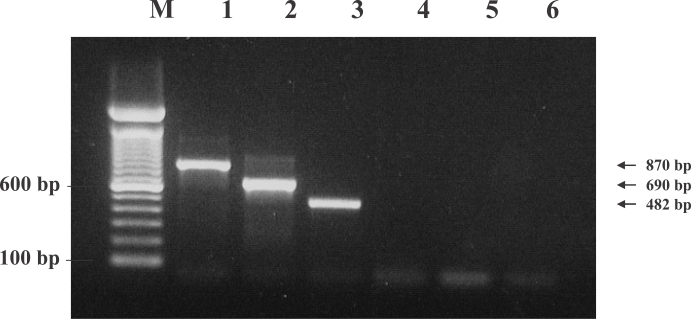
Fig. 5). Each PCR product was acquired only if the parasite DNA and its specific primers had been used (data not shown). This is, to the best of our knowledge, the first report to verify the existence of 2 types of hookworms and
Trichostrongylus sp. in Laotians using molecular biological techniques and genetic markers. Uterine eggs were extracted from adult female worms with a stereomicroscope, and employed in species-specific PCR without genomic DNA purification. The results were essentially the same as the above, and 3 eggs each were utilized to acquire the PCR products (data not shown).
There has been a report showing populational variations in the ITS-2 rDNA sequences of
N. americanus (
Romstad et al., 1998) between Africa and Malaysia. The extent of the differences between the ITS-2 sequences of
N. americanus from Africa and Malaysia was determined to be 1.8%. This sequence variation within species would be expected among
Trichostrongylus spp. and hookworms if more specimens were analyzed. Zhan et al. (
2001) described a PCR-based method for determining mitochondrial cytochrome oxidase I gene sequences rather than rDNA, and Gruijter et al. (
2005) demonstrated a PCR-based differential diagnosis of hookworm infections in Ghana. These results were comparable with the results of the present study, which indicated that the PCR approach is a valuable tool for the species-specific identification of human hookworms, or their infections.
In this study, no eggs were isolated from stool specimens. In the future, PCR can be applied in order to distinguish between hookworms and Trichostrongylus spp. eggs passed in the stool. Definitive identification can be achieved at the species level via PCR and nucleotide sequencing of Trichostrongylus spp., using even female worms or eggs, which are impossible to distinguish via morphological examinations. These techniques may prove useful in detailed studies of the epidemiology and population biology of Trichostrongylus spp. in endemic areas.
ACKNOWLEDGMENTS
The authors would like to thank the Korea Association of Health Promotion, Korea International Cooperation Agency and Ministry of Health, Lao PDR for their financial and human resources support.
References
Fig. 1
Trichostrongylus colubriformis adults collected from humans in Khamouane Province, Laos in 2003. (A) A female worm showing uterine eggs in one row. (B) Spicules of a male worm.

Fig. 2Phylogenetic relationship of the ITS regions from Trichostrongylus spp by using the PAUP* (version 4.0) program. The data were retrieved from GenBank except* T. colubriformis, of which was obtained in this study.
Fig. 3Nucleotide sequence alignment of the ITS-1 gene from Trichostrongylus colubriformis used in this study, Ancylostoma duodenale, and Necator americanus. The locations of the forward primers jmAD (5'-TGCGAAGTTCGCGTTTCGCTGAGCT-3'), jmNA (5'-CGTTAACATTGTATACCTGTACATAC-3'), and jhTsp (5'-TTATGTGCCACAAATGAAGA-3') are underlined in each line. Asterisks indicate identical nucleotides.

Fig. 4Schematic representation of part of the rDNA transcriptional unit and relative locations of primers (NC5, NC2, jmAD, jmNA and jhTsp) used in this study. The region harboring ITS 1, ITS 2, and 5.8 S rDNA was amplified with NC5 and NC2 primers. The species-specific primers, jmAD, jmNA, and jhTsp were targeted to the region within the ITS-1 sequence.
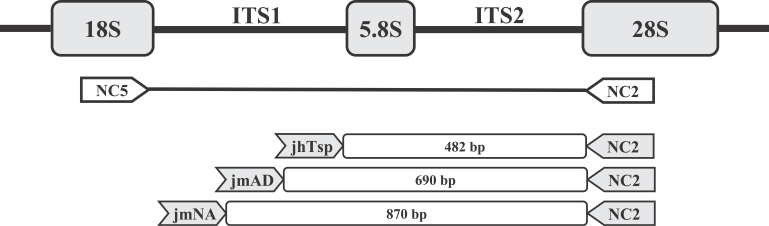
Fig. 5Specificity of ITS-PCR in the recognition of the genomic DNA of other bacteria, protozoa, Lane M, 100 bp ladder, 600 bp and 100 bp bands were shown. Lane 1; N. americanus, Lane 2; A. duodenale, Lane 3; Trichostrongylus colubriformis, Lane 4; Escherichia coli, Lane 5; Entamoeba histolytica, Lane 6; Giardia lamblia. Only specifically amplified PCR products were detected on lanes 1-3.