Abstract
Tegumental and excretory-secretory proteins are reported as diagnostic antigens for human opisthorchiasis. Rhophilin associated tail protein1-like (OvROPN1L) protein of Opisthorchis viverrini sperm tail showed potential as a diagnostic antigen. The OvROPN1L recombinant fragments were assayed for diagnostic antigenicity for human opisthorchiasis using indirect ELISA. The strongest antigenic region was a N-terminus peptide of M1 - P56. One synthetic peptide (P1, L3-Q13) of this region showed the highest antigenicity to opisthorchiasis. Sera from other parasitic infections including Strongyloides stercoralis, hookworm, Taenia spp, minute intestinal flukes, Paragonimus spp showed lower reactivity to P1. Peptide P1 is located in the disordered N-terminus of ROPN1L supporting its suitability as linear epitope. In the Platyhelminthes the N-terminal sequence of ROPN1L is diverging with taxonomic distance further suggesting that peptide P1 has potential as diagnostic tool in the genus Opisthorchis/Clonorchis. It should be further evaluated in combination with peptides derived from other O. viverrini antigens to increase its diagnostic power.
-
Key words: Opisthorchiasis, diagnosis, rhophilin associated tail protein1-like, peptide, epitope
The liver fluke
Opisthorchis viverrini causes cholangiocarcinoma, a cancer of the bile duct, in chronic infections. This cancer has a high incidence rate in the endemic areas in northeast Thailand [
1]. Early diagnosis of human opisthorchiasis can reduce incidence of the cancer. In human opisthorchiasis, the gold standard for diagnosis is microscopic detection and identification of
O. viverrini eggs from stool samples. The microscopic stool examination is dependent on experience of the examiner to differentiate helminth eggs, and is hampered in case of acute infections, light infections, or bile duct obstruction. Detection of parasite nucleic acids by PCR methods are interfered by abundant PCR inhibitors in the stools [
2–
4].
On opisthorchiasis diagnostics were searching for antigenic proteins in the tegumental and secretory-excretory products [
5–
7]. Interestingly, a sperm protein of
O. viverrini, rhophilin associated tail protein 1-like (OvROPN1L) protein was recognized by sera of
O. viverrini-infected humans and animals. OvROPN1L shows 47.2% sequence identity with the human homolog [
8].
In this study, we produced peptidic fragments of OvROPN1L (GenBank: KJ719301) and assayed them for diagnostic antigenicity for human opisthorchiasis.
Sera of human subjects with parasitic infections were collected in Pla Pak district, Nakhon Phanom Province, Thailand. Negative control sera of subjects without parasitic infections were collected in Pathumthani Province, Thailand. Stool samples of all subjects were prepared by formalin-acetic acid concentration technique and examined for parasite eggs by microscopy. Hamster sera were collected before (0 week) and after (12 weeks) infection with 50 metacercariae for each hamster (n=10).
The project protocol was approved by the Human Ethics Committee of Thammasat University following the International Conference on Harmonization–Good Clinical Practice (ICH–GCP), approval No. 132/2559. The animal protocol was approved by the Thammasat University Animal Ethics Committee, approval No. 024/2559.
The plasmid containing the complete CDS of OvROP1L was kindly provided by Dr. Suksiri Vichasri-Grams. The selected regions of the OvROPN1L cDNA were PCR-amplified using specific primers (
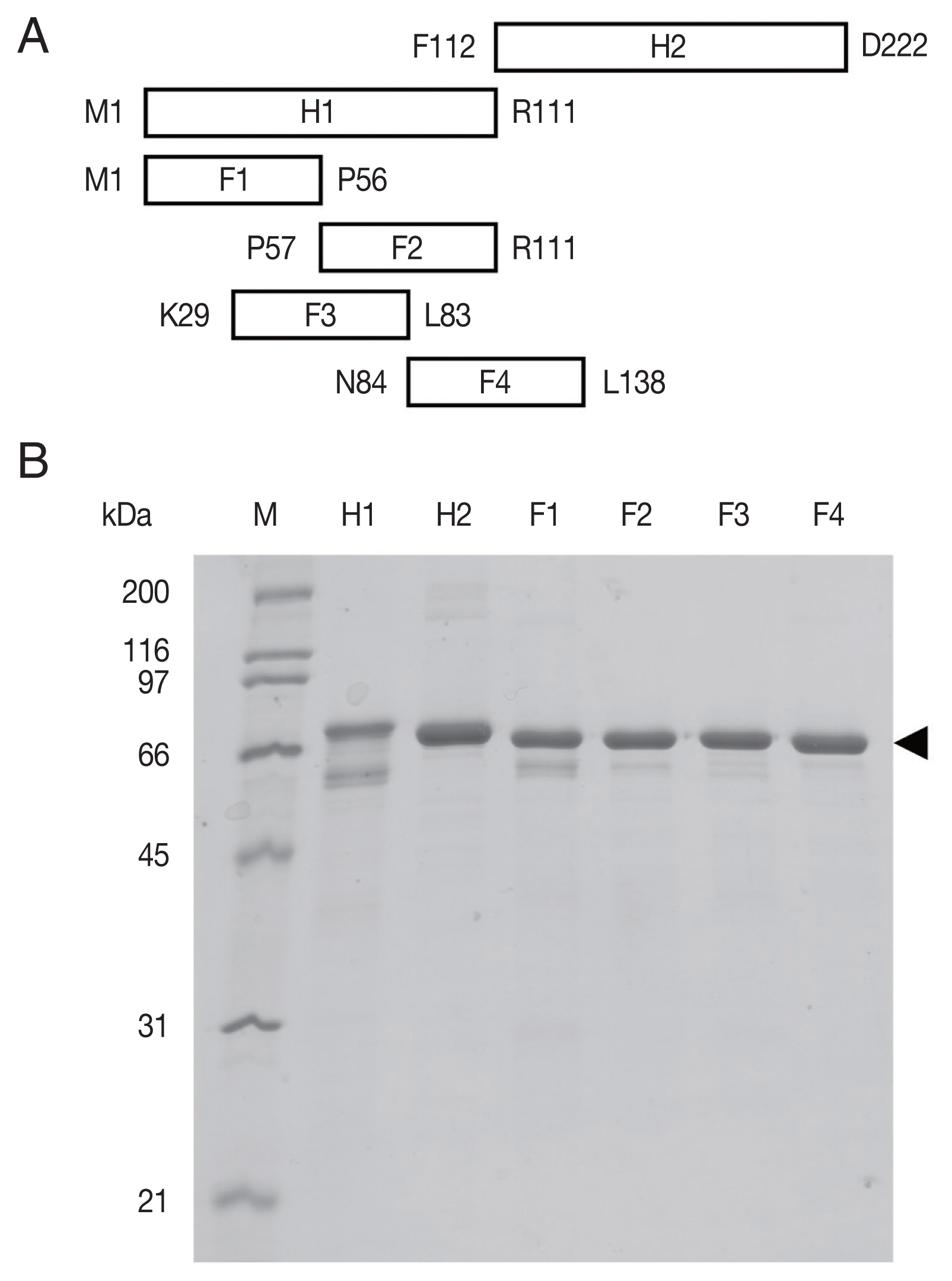
Supplement Table S1). The amplicons were fragments H1 comprising codons 1–111 and H2 codons 112–222, and 4 smaller fragments covering H1 (F1: codons 1–56, F2: codons 57–111, F3: codons 29–83, F4: codons 84–138). These cDNA fragments were subcloned into the vector pCold™ TF DNA (TaKaRa, Shiga, Japan). This plasmid vector produces a recombinant protein fused to trigger factor (TF). The expression host
E. coli BL21 was transformed with the recombinant plasmid DNA. The selected positive transformant
E. coli colonies were grown in terrific broth containing 100 μg/ml ampicillin at 16°C after IPTG induction. All expressed recombinant proteins were purified by Ni-NTA chromatography (QIAGEN, Hilden, Germany) under native conditions.
Recombinant OvROPN1L H1, H2, and F1–F4 in fusion with TF were cleaved using factor Xa (NEB, Ipswich, MA, USA) to release the OvROPN1L peptides from TF. Cleavage reactions were prepared by mixing 1 μg of factor Xa with 10 μg of target protein in cleavage buffer and incubated at 20°C for 20 hr. The unpurified cleavage products (mixture of OvROPN peptide and TF) were analyzed by SDS-PAGE and used for indirect ELISA.
Microtiter plates were coated with 1 μg cleavage product in carbonate buffer, pH 9.6. The purified TF without fusion was used to obtain the baseline value. The wells were washed with washing buffer (TBS pH 7.5, 0.1% [v/v] Tween 20). The wells were blocked with 1% (w/v) skim milk in washing buffer. The pooled hamster sera, either normal or O. viverrini-infected (n=10, 12 weeks post infection) were added at a dilution of 1: 100. Goat anti-Syrian hamster IgG (H+L) AP-conjugated antibody (Abcam, Cambridge, Massachusetts, USA) was used at a dilution of 1:1,000. The p-Nitrophenylphosphate (pNPP) substrate (Sigma Aldrich, St. Louis, Missouri, USA) was added and incubated in the dark at room temperature for 2 hr. Absorbance was measured at 405 nm.
Reactivity of infected hamster sera was strongest to recombinant peptide F1. Thus B-cell epitopes were predicted on the F1 region using BepiPred [
9] and the strongest predicted epitopes were further analyzed as synthetic peptides. The putative antigenic peptides (P1: L3–Q13, P2: H11–K25, P3: K29–K41, P4: R48–E63) in the F1 region were synthesized by Mimotopes Co. (Victoria, Australia). A microtiter plate was coated with 2 μg peptide or 100 ng of recombinant OvROPN1L in carbonate buffer, pH 9.6. The wells were washed with washing buffer (TBS, pH 7.5, 0.1% [v/v] Tween 20). The wells were blocked with 1% (v/v) bovine calf serum (Abcam). The normal or
O. viverrini-infected human sera (n=20 each) or other parasitic-infected human sera,
Strongyloides stercoralis (n=10),
Taenia spp. (n=3), hookworm (n=7), minute intestinal flukes (n=4),
Paragonimus spp. (n=3) were diluted 1: 100. Rabbit anti-human IgG HRP-conjugated antibody (Dako, Santa Clara, California, USA) was used at a 1:6,000 dilution. ABTS substrate (SeraCare, Milford, Massachusetts, USA) was added and the absorbance was measured at 405 nm.
Statistics of the results were evaluated using unpaired t-tests with GraphPad Prism 8. The experiments were performed in duplicate.
The recombinant OvROPN1L peptides H1, H2, F1–F4 (
Fig. 1A) in fusion with TF were expressed in a bacterial system and purified by Ni-NTA chromatography (
Fig. 1B). The calculated molecular weights of H1-TF and H2-TF fusion proteins were about 65 kDa (TF 52 kDa). The observed weights were slightly smaller than the calculated weights (
Fig. 1B). The fusion proteins of F1–F4 were also smaller.
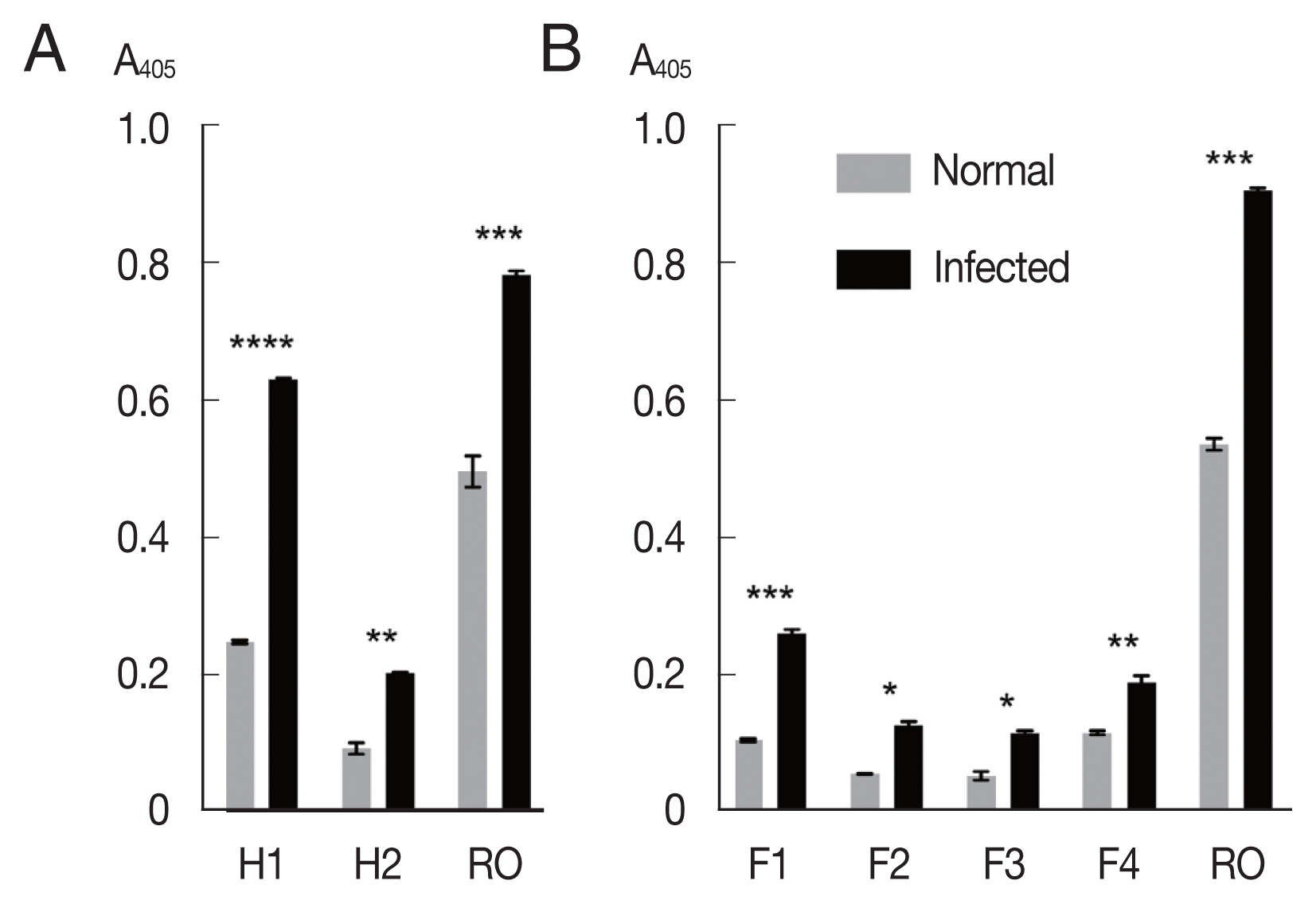
The mixtures of OvROPN1L peptides and TF were probed with pooled
O. viverrini-hamster sera (
Fig. 2A). The peptide H1 showed significantly higher absorbance values to the
O. viverrini-infected sera (
P<0.0001). The H2 peptide showed less difference to the infected/uninfected sera (
P=0.0054). The recombinant OvROPN1L was used as a positive control (
P= 0.0002). Antigenicity of the 4 shorter recombinant peptides F1–F4 were assayed with the same hamster sera. The antigenicity of peptide F1 was highest (
P=0.0026,
Fig. 2B).
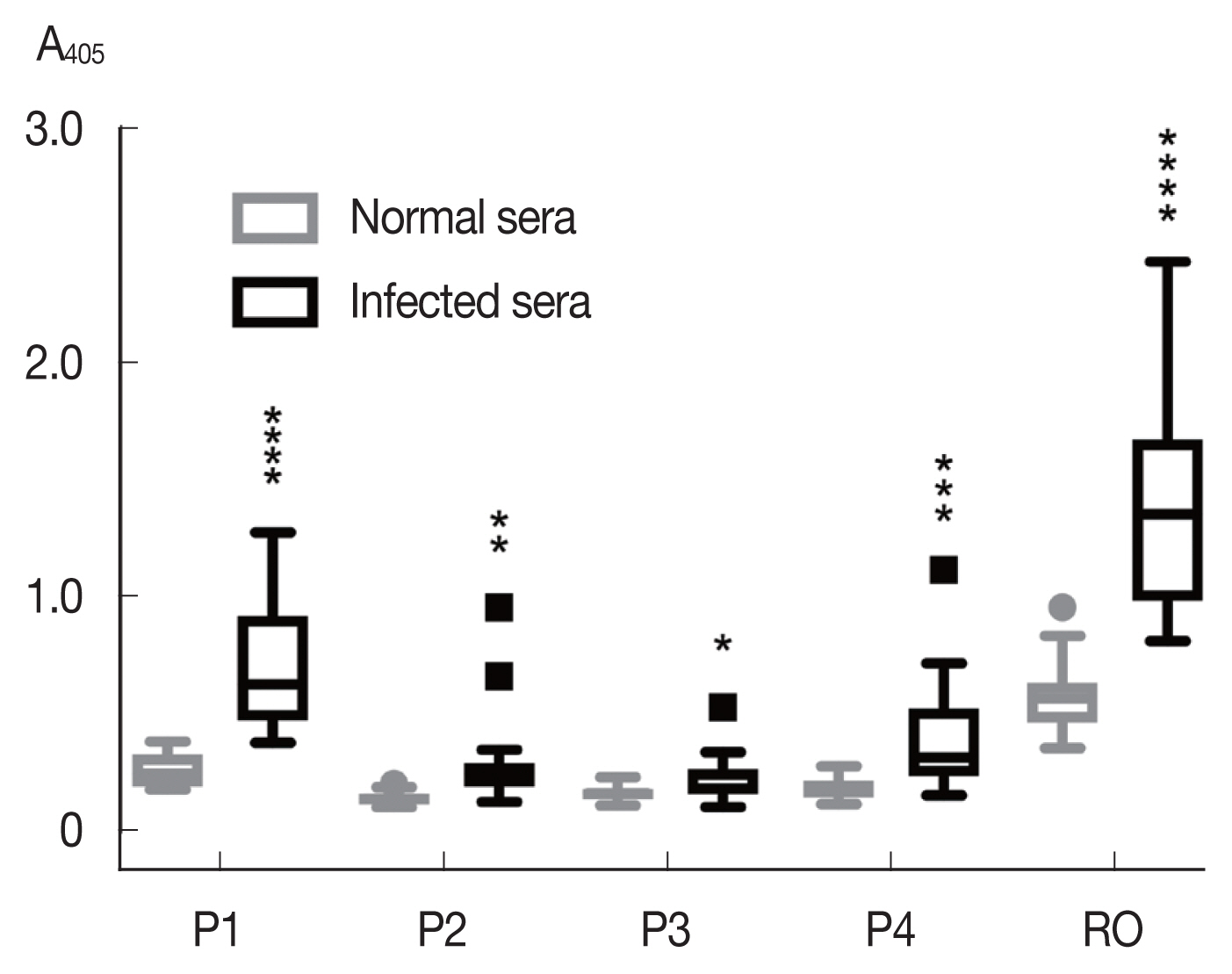
Among the synthetic peptides (P1–P4) of the 4 predicted B-cell epitopes assayed with human opisthorchiasis sera, the peptide P1 showed highest antigenicity (
Fig. 3), indicating that peptide P1 had potential as diagnostic antigen.
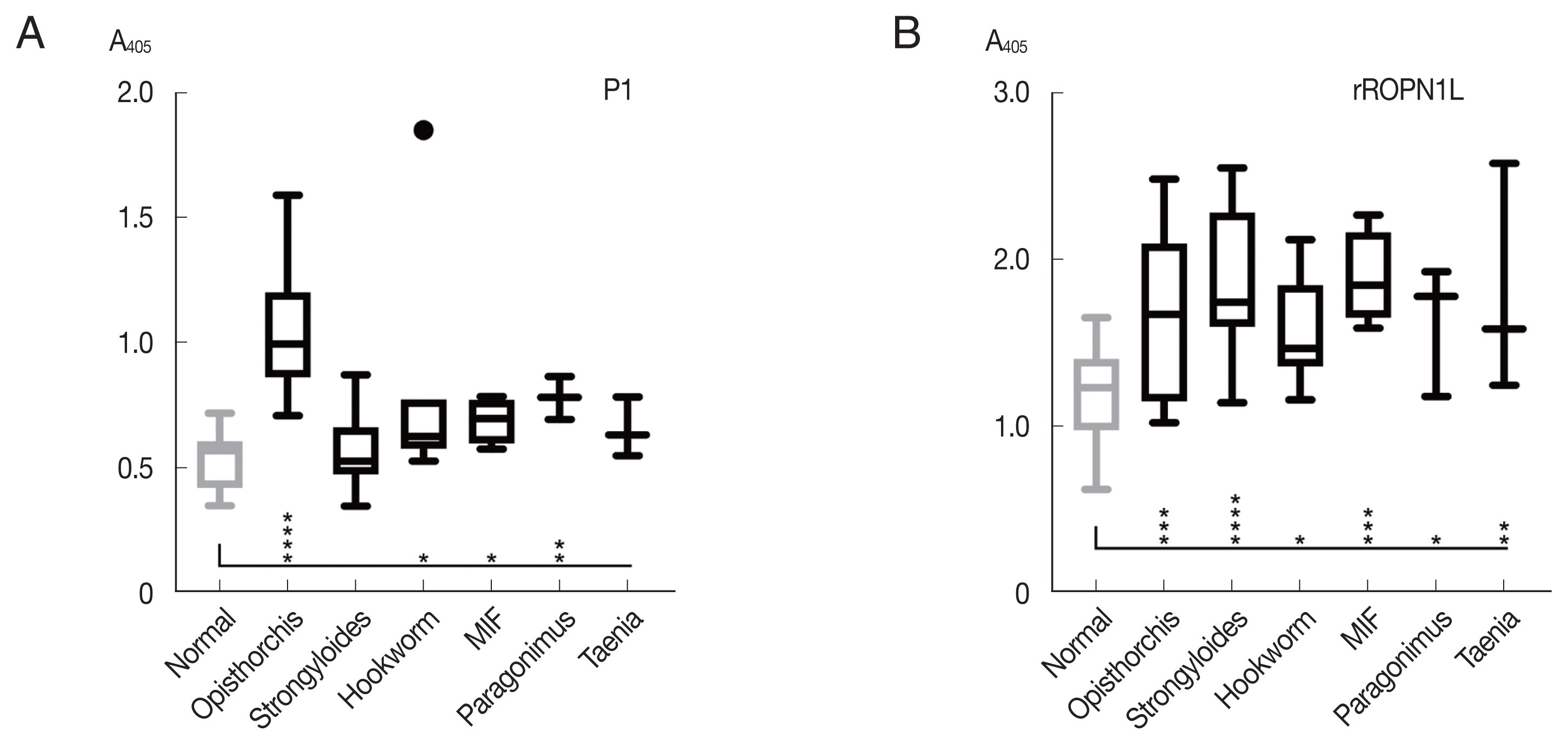
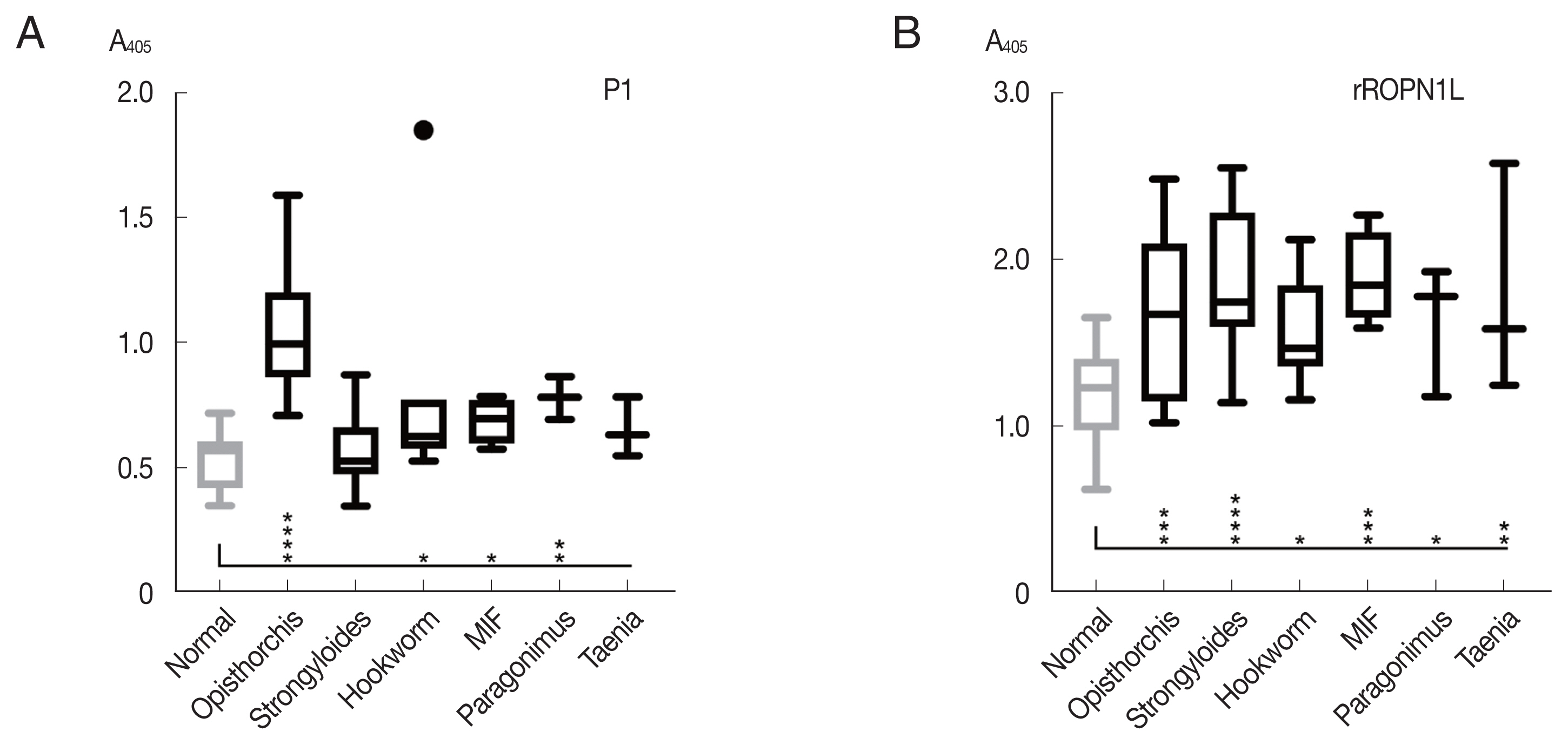
The peptide P1 was further analyzed with helminth-infected sera, including
Strongyloides stercoralis,
Taenia spp.,
Paragonimus spp., hookworm, and minute intestinal flukes in comparison with complete recombinant OvROPN1L. The peptide P1 showed no statistically significant reactivity toward the human sera of other helminth infections, while the ROPN1L showed high cross-reactivities (
Fig. 4). In case of peptide P1 the absorbance values of
O. viverrini-infected sera were significantly higher than the other helminth-infected sera.
In mammals, rhophilin-associated tail protein has been investigated how it is involved in the cAMP-dependent protein kinase pathway, and how to interact with A-kinase anchor protein, and how it affects sperm motility [
10–
12]. In trematodes, only OvROPN1L has been molecularly characterized [
8]. OvROPN1L was already expressed in 2-week-old juveniles which could be an advantage if it can be applied for diagnosis [
8]. In this study, we identified an immunodominant epitope of OvROPN1L that might be applicable for diagnosis of human opisthorchiasis. The most sensitive epitope (peptide P1, L1–Q13, LVNDPYYCHEQ) was located at the protein’s N-terminus. The peptide P1 was comparable to full OvROPN1L and showed higher specificity for detection of
O. viverrini infection but less sensitivity. Structural analysis in Jpred4 [
13] and Phyre2 [
14] predicted a mostly disordered structure of L3–Q13 (P1) while regions representing P2–P4 had higher helical content and a more complex folding in the ROPN1L model. BLASTP revealed N-terminal sequence divergence of ROPN1L in the Platyhelminthes (
Supplementary Fig. S1).
Paragonimus westermani with 3 amino acid differences in P1 (the highest scoring match outside the genus
Opisthorchis/
Clonorchis) already showed low reactivity with the
O. viverrini sera (
Fig. 4A). Thus, a next step in the follow-up study could be further refinement of the peptide P1 using more sera. Serology can provide additional insight and complement microscopy in diagnosis of the helminthic infections [
15]. Immunodiagnostic assays must evaluate cross reactivity with other parasite infections including helminths and protozoans to validate the results.
In conclusion, the N-terminal peptide P1 (L3–Q13) was assayed as a diagnostic antigenic epitope of OvROPN1L. However, the exact epitope in P1 needs to be identified in the future to determine whether its specificity can be increased.
Supplementary Information
Notes
-
CONFLICT OF INTEREST
The authors declare that there are no conflicts of interest.
ACKNOWLEDGMENTS
This research was financially supported by a grant through Thammasat University, contract no 34/2561.
Fig. 1The recombinant rhophilin associated tail protein 1-like (OvROPN1L) peptides of Opisthorchis viverrini. (A) Schematic drawing of the 6 regions (H1, H2, F1, F2, F3, and F4) of OvROPN1L expressed in E. coli as recombinant peptides. The terminal amino acids of both ends are indicated. (B) SDS-PAGE of peptides H1, H2, F1–F4 fused with trigger factor. The soluble fusion proteins were purified under native condition by Ni-NTA affinity chromatography. A black arrow indicates fusion proteins.
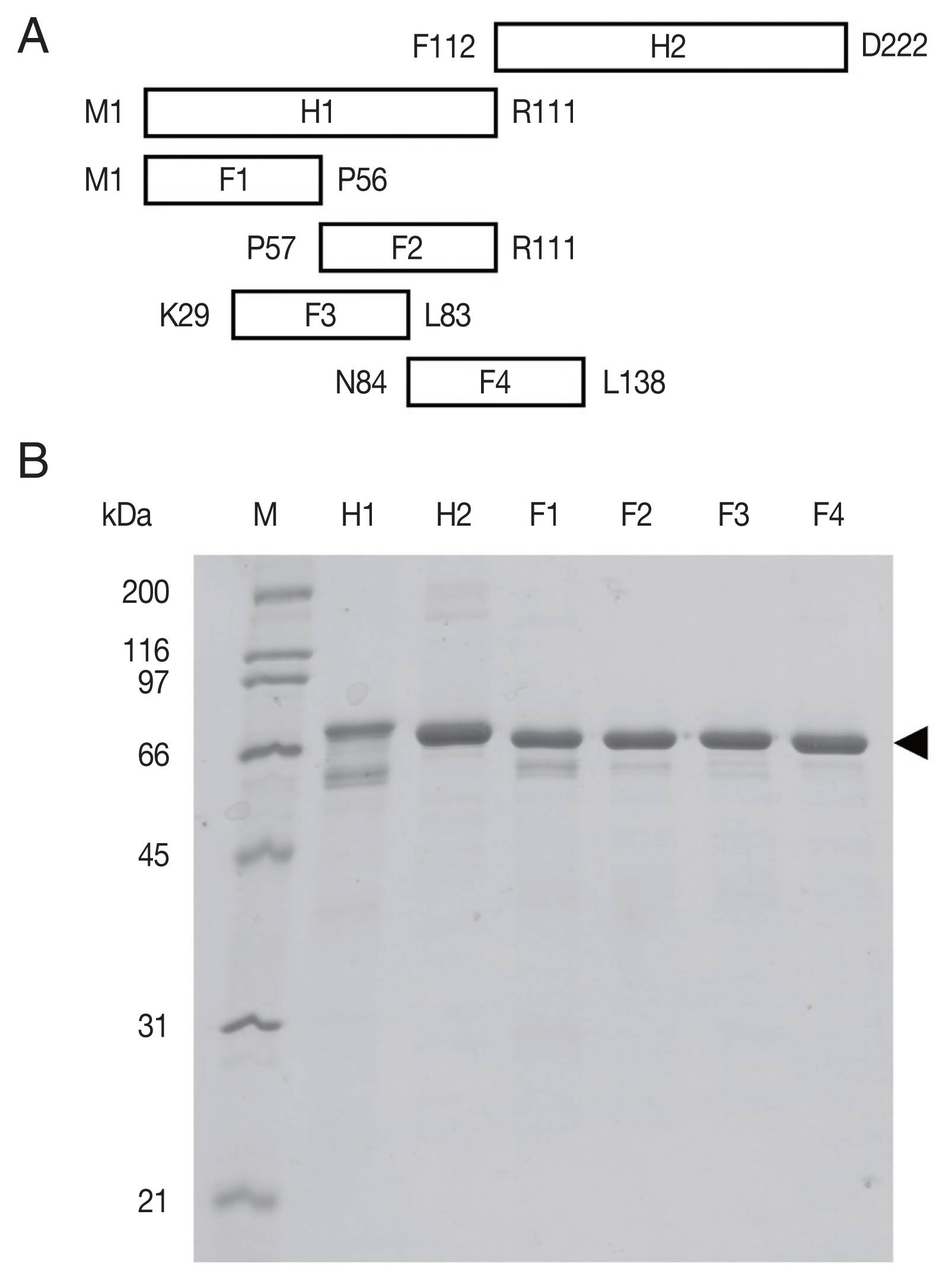
Fig. 2Antigenicity of recombinant proteins (H1, H2, F1, F2, F3, F4, and OvROPN1L) toward the pooled O. viverrini-infected hamster serum using indirect ELISA. Statistical significance between infected and uninfected sera, *P<0.05, **P<0.001, ***P<0.0001, ****P<0.00001.
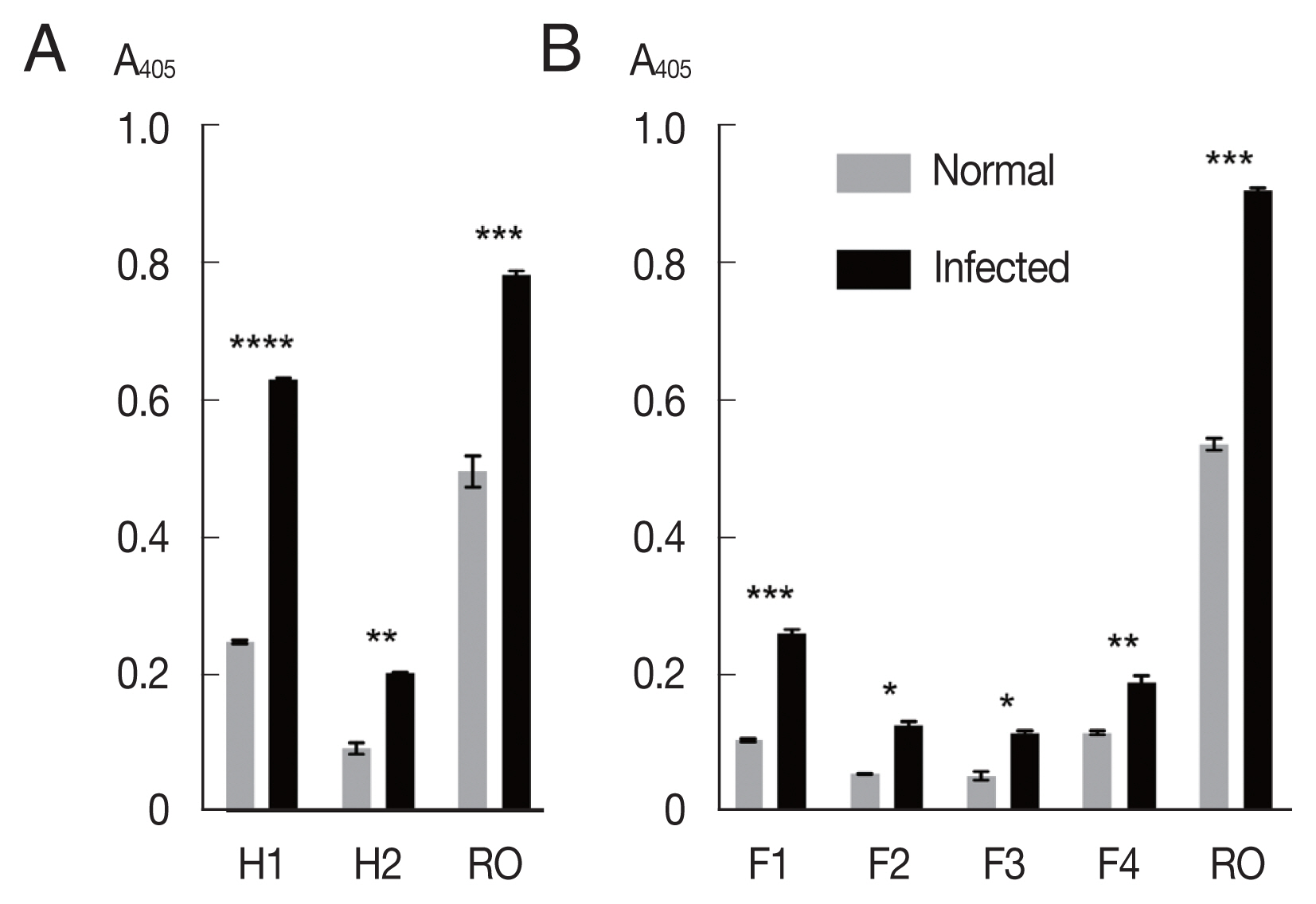
Fig. 3Antigenicity (mean with SD) of synthetic peptides against human opisthorchiasis sera (n=20) using indirect ELISA. Statistical significance between infected and uninfected sera, *P<0.05, **P<0.001, ***P<0.0001, ****P<0.00001.
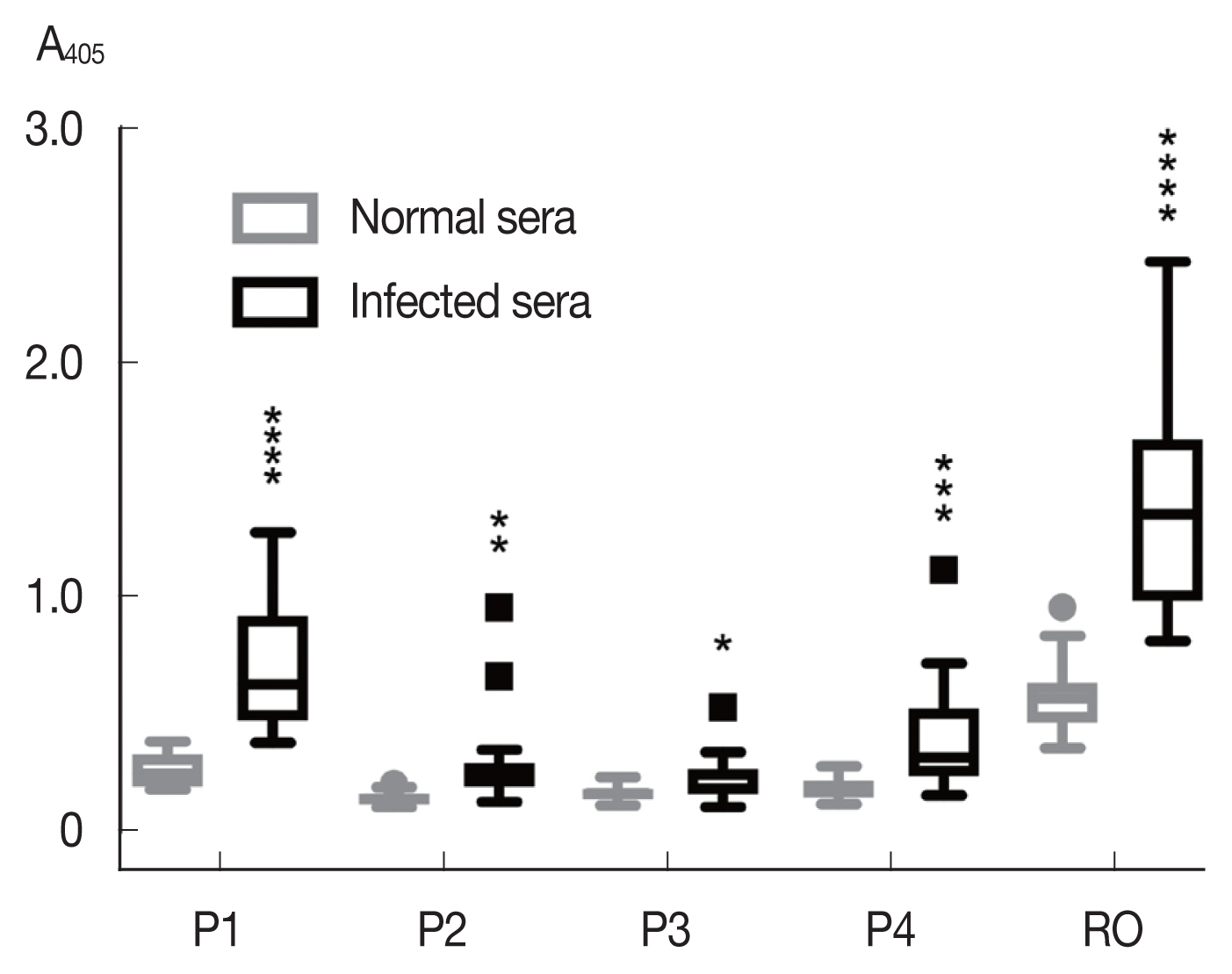
Fig. 4Reactivity (mean with SD) of synthetic peptide P1 using indirect ELISA. (A) Synthetic peptide 1. (B) Recombinant OvROPN1L. Statistical significance between infected and uninfected sera, *P<0.05, **P<0.001, *** P<0.0001, ****P<0.00001.
References
- 1. Sripa B, Bethony JM, Sithithaworn P, Kaewkes S, Mairiang E, Loukas A, Mulvenna J, Laha T, Hotez PJ, Brindley PJ. Opisthorchiasis and opisthorchis-associated cholangiocarcinoma in Thailand and Laos. Acta Trop 2011;120(suppl):158-168.
- 2. Umesha KR, Kumar S, Parvathi A, Duenngai K, Sithithaworn P, Karunasagar I, Karunasagar I.
Opisthorchis viverrini: detection by polymerase chain reaction (PCR) in human stool samples. Exp Parasitol 2008;120:353-356.
- 3. Sato M, Pongvongsa T, Sanguankiat S, Yoonuan T, Dekumyoy P, Kalambaheti T, Keomoungkhoun M, Phimmayoi I, Boupha B, Moji K, Waikagul J. Copro-DNA diagnosis of Opisthorchis viverrini and Haplorchis taichui infection in an endemic area of Lao PDR. Se Asian J Trop Med 2010;41:28-35.
- 4. Duenngai K, Boonmars T, Sithithaworn J, Sithithaworn P. Diagnosis of early infection and post chemotherapeutic treatment by copro-DNA detection in experimental opisthorchiasis. Parasitol Res 2013;112:271-278.
- 5. Worasith C, Kamamia C, Yakovleva A, Duenngai K, Wangboon C, Sithithaworn J, Watwiengkam N, Namwat N, Techasen A, Loilome W, Yongvanit P, Loukas A, Sithithaworn P, Bethony JM. Advances in the Diagnosis of Human Opisthorchiasis: development of Opisthorchis viverrini antigen detection in Urine. PLoS Negl Trop Dis 2015;9:e0004157.
- 6. Teimoori S, Arimatsu Y, Laha T, Kaewkes S, Sereerak P, Sripa M, Tangkawattana S, Brindley PJ, Sripa B. Chicken IgY-based coproantigen capture ELISA for diagnosis of human opisthorchiasis. Parasitol Int 2017;66:443-447.
- 7. Jamornthanyawat N. The diagnosis of human opisthorchiasis. Southeast Asian J Trop Med Public Health 2002;33(suppl):86-91.
- 8. 8Rattanachan S, Grams R, Tesana S, Smooker PM, Grams SV.
Opisthorchis viverrini: analysis of the sperm-specific rhophilin associated tail protein 1-like. Acta Trop 2014;140:34-40.
- 9. Larsen JE, Lund O, Nielsen M. Improved method for predicting linear B-cell epitopes. Immunome Res 2006;2:1-7.
- 10. Xu KB, Qi HY. Sperm-specific AKAP3 is a dual-specificity anchoring protein that interacts with both protein kinase a regulatory subunits via conserved N-terminal amphipathic peptides. Mol Reprod Dev 2014;81:595-607.
- 11. Carr DW, Fujita A, Stentz CL, Liberty GA, Olson GE, Narumiya S. Identification of sperm-specific proteins that interact with A-kinase anchoring proteins in a manner similar to the type II regulatory subunit of PKA. J Biol Chem 2001;276:17332-17738.
- 12. Fiedler SE, Dudiki T, Vijayaraghavan S, Carr DW. Loss of R2D2 proteins ROPN1 and ROPN1L causes defects in murine sperm motility, phosphorylation, and fibrous sheath integrity. Biol Reprod 2013;88:41.
- 13. Drozdetskiy A, Cole C, Procter J, Barton GJ. JPred4: a protein secondary structure prediction server. Nucleic Acids Res 2015;43:W389-394.
- 14. Kelley LA, Mezulis S, Yates CM, Wass MN, Sternberg MJ. The Phyre2 web portal for protein modeling, prediction and analysis. Nat Protoc 2015;10:845-858.
- 15. Ricciardi A, Ndao M. Diagnosis of parasitic infections: what's going on? J Biomol Screen 2015;20:6-21.
 , Rudi Grams1, Wansika Phadungsil1, Wanlapa Chaibangyang1,2, Nanthawat Kosa1, Poom Adisakwattana3, Paron Dekumyoy3
, Rudi Grams1, Wansika Phadungsil1, Wanlapa Chaibangyang1,2, Nanthawat Kosa1, Poom Adisakwattana3, Paron Dekumyoy3