Abstract
Monoclonal antibody (mAb) Tg786 against Toxoplasma gondii has been found to detect a 42-kDa rhoptry protein (ROP6) which showed protease activity and host cell binding characteristics after secretion. Using the mAb, a colony containing a 3'-UTR was probed in a T. gondii cDNA expression library. A full length cDNA sequence of the rhoptry protein was completed after 5'-RACE, which consisted of 1,908 bp with a 1,443 bp ORF. The deduced amino acid sequence of ROP6 consisted of a polypeptide of 480 amino acids without significant homology to any other known proteins. This sequence contains an amino terminal stop transfer sequence downstream of a short neutral sequence, hydrophilic middle sequence, and hydrophobic carboxy terminus. It is suggested that the ROP6 is inserted into the rhoptry membrane with both N- and C-termini.
-
Key words: Toxoplasma gondii, excretory/secretory proteins, ROP6, cDNA sequence, hydrophilic domain
Among the 3 proteases found in the excretory/secretory proteins (ESP) of
Toxoplasma gondii, a 42 kDa protease was immunoprecipitated by a monoclonal antibody (mAb), Tg786, which detected the protein in the rhoptry organelles of
T. gondii by immunofluorescence assay (
Ahn et al., 2001). The secreted protease targeted to the plasma membrane of host cells, which was suggested to favor the appropriate environment for the entry of the parasite into host cells. Using the mAb, a
T. gondii cDNA expression library was screened in order to obtain the genetic information of the 42 kDa rhoptry protease.
A T. gondii λ ZAPII cDNA expression library obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, National Institutes of Health (McKesson Biosciences, Rockville, Maryland, USA) was screened in Escherichia coli XL1- Blue MRF' (Stratagene, La Jolla, California, USA) using mAb Tg786 in PBS/Tween-20 containing 1% (w/v) BSA. Positive plaques were detected using an ECL Detection System (Amersham Phamacia Biotech, Uppsala, Sweden). pBluescript SK phagemids were isolated by co-infection of the λ ZAPII phage and the ExAssist helper phage (Stratagene). The excised phagemids were further propagated in the E. coli SOLR host strain (Stratagene) to purify the phagemid DNA. All DNA sequencing was performed using a dye terminator fluorescent-based sequence analysis on an Applied Biosystems 373 automated sequencer using primers directed to the vector T7 and T3 promoter sequences. The longest cDNA clone containing a poly (A+) tail was selected for the design of the internal gene-specific primers (Bioneer Co., Daejeon, Korea).
The RH strain of
T. gondii was maintained via peritoneal passage in BALB/c mice. Prior to use, tachyzoites were purified by centrifugation over 40% Percoll (Amersham Phamacia Biotech) in PBS solution. Total
T. gondii tachyzoite RNA was extracted using Tri reagent (Sigma Chemical Co., St. Louis, Missouri, USA) for the 5'-RACE procedure (
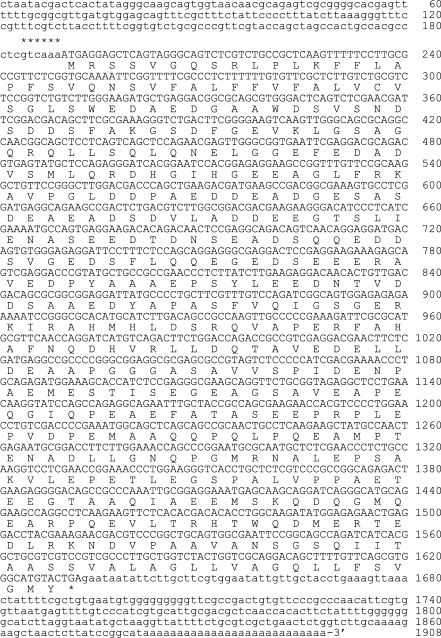
Frohman et al., 1998). First strand cDNA was synthesized from 1 µg of total RNA by using a Superscript Preamplification System (Life Technologies, Gaithersburg, Maryland, USA). DeoxyCTPs were added to the 3' end of the non-coding cDNA using terminal deoxynucleotide transferase (Life Technologies). PCR amplification of C-tailed cDNA was performed with an anchor primer (5'-CTA ATA CGA CTC ACT ATA GGG CAA GCA GTG GTA TCA ACG CAG AGT-3') and a gene-specific primer (5'-CGT TCG AGA CTT GAG TCC CAG GCT-3'). The first-round product was then amplified further with the abridged universal anchor primer (5'- AAG CAG TGG TAT CAA CGC AGA GT-3') coupled with an internal gene-specific primer (5'-GCG AAA ACC GAA TTT TGC ACC GAG-3'). The secondround PCR product was cloned into the pGEM-T EASY vector (Promega, Madison, Wisconsin, USA) to sequence using T7 and SP6 primers. The complete 1,908 bp sequence was constructed by the 5'-RACE method containing an 1,443 bp open reading frame (
Fig. 1). A search for homologous sequences in the
Toxoplasma dbEST (Database of Expressed Sequence Tags) using the BLASTn algorithm with default settings resulted in the match of a contig assembly containing 20 ESTs with high BLAST scores. A full cDNA sequence was registered in the GenBank (Accession No. AY792971) as a 42 kDa rhoptry protein (ROP6) of
T. gondii. Using the second in-frame ATG as a starting site downstream of the typical Tg consensus translation initiation sequence of GTCAAA (
Seeber, 1997), the ROP6 gene encoded a polypeptide of 480 amino acids with a molecular mass of 42 kDa. The protein sequence was then compared to entries in the GenBank database using BLASTp search, which resulted in no matches to any previously known proteins. The hydropathicity of the amino acids sequence was obtained from ExPASy using the Kyte and Doolittle (
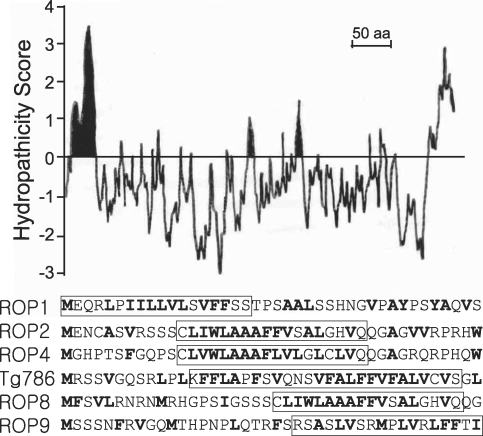
1982) calculation. The sequence contains amino terminal stop transfer sequence downstream of a short neutral sequence, a long middle hydrophilic sequence, and a hydrophobic carboxy terminus (
Fig. 2), thereby suggesting that the ROP6 is inserted into the rhoptry membrane with both the N- and C-termini in a similar fashion to that of other ROP proteins except for ROP1.
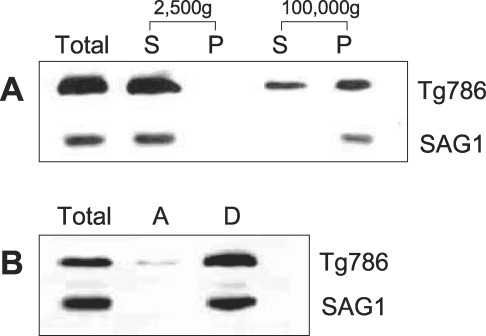
The membrane insertion of ROP6 was confirmed by differential centrifugation and the Triton X-114 phase partitioning (
Bordier, 1981) of the protein. When the supernatants of the tachyzoite extracts in PBS at 2,500 g centrifugation was sequentially centrifuged at 100,000 g, major parts of the protein was blotted with mAb Tg786 in the precipitant with a smudge in the supernatant (
Fig. 3A). The tachyzoite extracts in the extraction buffer (10 mM Tris-HCl, pH 7.4, 150 mM NaCl, 0.5% (v/v) precondensed Triton X-114 (Pierce, Rockford, Illinois, USA) and a 1 : 100 (v/v) dilution of aqueous and DMSO protease inhibitor stocks) were centrifuged at 13,000 g, which blotted the ROP6 in the detergent phase and not in the aqueous phase (
Fig. 3B). A major surface antigen of
T. gondii (SAG1) behaved as a control of the typical membrane inserting protein in both centrifugations.
Until now, 9 rhoptry proteins (ROP1 - ROP9) have been found to contribute to the formation of the parasitophorous vacuole (
Ngo et al., 2004). We have added the genetic information of ROP6 to the genes of ROP1, 2, 4, 8, and 9, but still no clues to the properties from the gene sequences or deduced amino acid sequences, even protease moiety of ROP6. These are similar to other secretory proteins from secretory organelles, such as, the MIC proteins of micronemes and the GRA proteins of dense granules. ROP proteins are potent antigens that can induce strong parasite-directed Tcell and B-cell responses (
Reichman et al., 2002). Therefore, ROPs deserve to be considered as promising candidates for vaccine development. ROP6 appears to constitute an excellent candidate for a potential vaccine, because it is secreted as ESP and exhibits protease activity essential for the entry of the parasite into host cells.
Notes
-
This work was supported by the Korea Research Foundation Grant funded by the Korean Government (MOEHRD) (KRF-2005-041-E00128).
References
Fig. 1The cDNA and deduced amino acid sequences of the ROP6 of Toxoplasma gondii. Nucleotide sequence: the sequence of ROP6 contains an open reading frame of 1,443 bp downstream of the Tg consensus translation initiation sequence of gtcaaa, as indicated by stars. The nucleotide numbers are shown on the right. Nucleotide sequence is available in the GenBank under the accession number of AY792971, and the deduced amino acid sequence: the ORF of 1,443 bp encodes a polypeptide of 480 amino acids.

Fig. 2Hydropathicity of ROP6 sequence and the N'-terminal amino acid sequences of ROP proteins. The internal hydrophilic sequence is lodged in the rhoptry membrane in both the N'- and C'-termini. Potential stop transfer sequences (the signal sequence in case of the ROP1) are indicated with boxes.

Fig. 3Physical sedimentation of RH tachyzoite extracts (A) and Triton X-114 phase partitioning of the extracts (B). S: supernatant of RH tachyzoite extracts after centrifugation, and P: precipitant. Total parasite proteins (lane 1), A: proteins recovered in the aqueous phase (lane 2), and D: detergent (lane 3) phase after partitioning were blotted with by mAb Tg786 and mAb to SAG1 as a control.

Citations
Citations to this article as recorded by

- A systematic review of Toxoplasma gondii antigens to find the best vaccine candidates for immunization
Fatemeh Rezaei, Shahabeddin Sarvi, Mahdi Sharif, Seyed Hossein Hejazi, Abdol sattar Pagheh, Sargis A. Aghayan, Ahmad Daryani
Microbial Pathogenesis.2019; 126: 172. CrossRef - Toxoplasma gondii Secretory Proteins and their Role in Invasion and Pathogenesis
Yang Zhang, Bo Shiun Lai, Mario Juhas, Yun Zhang
Microbiological Research.2019;[Epub] CrossRef - A systematic review on the role of GRA proteins of Toxoplasma gondii in host immunization
Fatemeh Rezaei, Mahdi Sharif, Shahabeddin Sarvi, Seyed Hossein Hejazi, Sargis Aghayan, Abdol Sattar Pagheh, Samira Dodangeh, Ahmad Daryani
Journal of Microbiological Methods.2019; 165: 105696. CrossRef - Rhoptry protein 6 from Toxoplasma gondii is an intrinsically disordered protein
Won-Kyu Lee, Hye-Jin Ahn, Yeon Gyu Yu, Ho-Woo Nam
Protein Expression and Purification.2014; 101: 146. CrossRef - Electrophoretic Patterns of Toxoplasma gondii Excreted/Secreted Antigens and Their Role in Induction of the Humoral Immune Response
Ahmad Daryani, Mehdi Sharif, Hamed Kalani, Alireza Rafiei, Farzad Kalani, Ehsan Ahmadpour
Jundishapur Journal of Microbiology.2014;[Epub] CrossRef - Kiss and spit: the dual roles of Toxoplasma rhoptries
John C. Boothroyd, Jean-Francois Dubremetz
Nature Reviews Microbiology.2008; 6(1): 79. CrossRef - Characterization of a New Gene <italic>wx2</italic> in <italic>Toxoplasma gondii</italic>
Xiang Wu, Qiong Zhang, Kui Tan, Ronghua Xie, Jiubo Fan, Hengping Shu, Shiping Wang
Acta Biochimica et Biophysica Sinica.2007; 39(7): 475. CrossRef