Abstract
The present study reports a rare case of Taenia saginata infection, which was initially diagnosed as acute cholecystitis in a Tibetan patient at the Qinghai-Tibetan Plateau pastoral area, China. A 45-year-old female was initially diagnosed with acute cholecystitis at a hospital in China. She had a slight fever, weight loss and constipation and complained of pain in the upper abdomen and left back areas. Increase of monocyte, eosinophil and basophil levels were shown. Taenia sp. eggs were detected in a fecal examination. An adult tapeworm approximately 146 cm in length, whitish-yellow color, was collected from the patient after treatment with traditional Chinese medicine. The adult tapeworm had a scolex and proglottids with genital pores. The scolex was rectangular shape with 4 suckers and rostellum without hooklet. The cox1 gene sequence shared 99.5–99.8% homology with that of T. saginata from other regions in China. The patient was diagnosed finally infected with T. saginata by morphological and molecular charateristics.
-
Key words: Taenia saginata, cox1 gene, Qinghai-Tibet Plateau, Pastoral area, Tibetan
Taeniasis is a neglected foodborne tropical disease caused by
Taenia solium,
Taenia saginata, and
Taenia asiatica infection; these parasites have a worldwide distribution. The metacestode larval stage (cysticercus) parasitizes the intermediate host and causes cysticercosis. This type of parasite infection usually occurs when people eat fresh or undercooked pork or beef containing cysticerci; therefore, it is also called “food-borne parasitosis”. Based on the detection of tapeworm eggs in the faeces of people by the improved Kato-Katz method in 31 provinces in China from 2014 to 2015 [
1], the prevalence of tapeworm infection in China was 0.36%, and the ecological area with the highest infection rate was the area including eastern Tibet and southeastern Sichuan Province. Specifically, 94.69% of infected people were from the Tibet Autonomous Region, where the infection rate was 9.25%; the infection rate in the Tibet Autonomous Region was approximately 24.7 times higher than the national average infection rate. According to the results of the first national parasite investigation in 1990, the infection rates of
T. saginata in Maqin County of Guoluo Prefecture and Xiewu town of Yushu Prefecture in the pastoral area of Qinghai Province were 3.50% and 3.40%, respectively [
2]. In the parasite survey conducted in 2014–2015, the tapeworm infection rate in Qinghai Province was 0.01%, ranking 4th in the country, but it was 90.9% lower than the rate observed in the second investigation (0.11%) in 2004 [
3]. In 2015, taeniasis and cysticercosis were listed among 17 neglected tropical diseases in WHO report [
4], and approximately 100 million people were reported to be infected with
T. saginata worldwide [
5]. In a review, it was shown that the prevalence of
T. saginata infection was 3.8%, 4.7%, 1.6%, 0.5%, 4.4%, 7.0%, 33.7%, and 0.3% in humans in India, Indonesia, Lao PDR, Mongolia, Nepal, Pakistan, Philippines and Thailand, respectively [
6], and
T. saginata infection cases were also reported in Myanmar [
7].
According to an epidemiological survey of taeniasis in the Tibetan areas of Sichuan Province adjacent to Qinghai in 2013, there were 3
Taenia epidemics in the region, and
T. saginata was the dominant species [
8]. According to a retrospective survey of 12 patients (Goulo Tibetans) with taeniasis admitted to the affiliated hospital of Qinghai University in 2007, 3 patients were infected with
T. saginata and 9 patients were infected with
T. solium [
9].
Recently, many immunological and molecular diagnosis methods have been developed. Although it is difficult to distinguish between
T. solium and
T. saginata eggs with identical morphology, the infectious agent can be identified as
Taenia spp. The immunological diagnosis method ELISA also cannot distinguish between the 2 species. Molecular diagnostic methods are becoming increasingly used to distinguish among different species of tapeworms [
10–
12]. Many antimicrobial drugs (such as niclosamide and praziquantel) are used to treat
T. saginata infections in humans, and vaccines and biological control methods are also available [
13]. In this report, we describe a patient infected with
T. saginata cured by traditional Chinese medicine, the species was confirmed by molecular analysis of the worm.
The patient was a 45-year-old female Tibetan herder from Dari County of Guoluo Prefecture of the Qinghai Province pastoral area. She was in close contact with cattle, sheep and dogs. She also had a habit of eating raw yakiniku and air-dried meat but reported no elimination of gravid proglottids; she had not travelled to other parts of China or abroad. The patient reported a slight high fever and development of pain in her upper abdomen, intermittent pain and discomfort under the xiphoid, and radiating pain in the left back but no nausea, vomiting or loss of appetite; however, she did experience recent significant weight loss and symptoms of constipation. After a medical examination, the pain was determined to be located in the right upper abdomen and under the xiphoid at 3 horizontal finger lengths below the ribs, and there were no lumps in the abdomen. The gallbladder was positive for Murphy’s sign. Therefore, the patient was initially diagnosed with acute cholecystitis and was admitted to the hospital for treatment.
B-ultrasound examination of the abdomen was performed to determine the imaging indications of the liver and gallbladder. Whole blood was drawn for routine blood tests, including evaluation of the eosinophil and basophil indexes associated with parasitic infections and biochemical indicators in the serum, such as the total protein and albumin levels. The patient’s stool was collected for an occult blood test, and parasite egg examination was also performed. The traditional Chinese medicine “pumpkin seed (Nan Guazi)-betel nut (Binglang)” method was used for parasite deworming, as described in the literature. The tapeworm was collected, morphological analysis was also performed, and the gravid proglottid was stained with haematoxylin and eosin.
A portion of the worm was used in the molecular analysis. Total genomic DNA was extracted from the tapeworm using a DNeasy
® Blood & Tissue Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions, and the DNA was stored at −20°C. PCR targeting the tapeworm
cox1 gene was performed as previously described [
14]. The primers used for the PCR were
cox1-F: 5′-TTT TTT GGG CAT CCT GAG GTT TAT-3′ and
cox1-R: 5′-TAA AGA AAG AAC ATA ATG AAA ATG-3′, which generated a product of approximately 440 bp. PCR was performed in standard mixtures of 50.0 μl containing 4.0 μl of primer mixtures (10.0 μM each primer), 25.0 μl of 2× PCR SuperMix, 3.0 μl of DNA, and 18.0 μl of PCR-grade water. The PCR program was performed in a Mastercycler Nexus GSX1 (Eppendorf, Germany) as follows: initial heat activation step at 94°C for 5 min; 35 cycles of 94°C for 30 sec, 54°C for 30 sec, and 72°C for 30 sec; a final extension at 72°C for 10 min; and a final hold at 4°C. A positive control and negative control were included in the amplification. The amplified PCR products were analysed using a 1.5% agarose gel and were observed under UV light. The positive PCR products were sequenced by SUZHOU GENEWIZ Company (Suzhou, China) with the Sanger sequencing method. Homologous sequence alignment of the
cox1 gene was carried out with BLAST (
https://blast.ncbi.nlm.nih.gov/Blast.cgi), and homology analysis on the basis of reference sequences was conducted with Clustal Omega (
http://www.ebi.ac.uk/Tools/msa/clustalo/). Phylogenetic analysis of
Taenia cestodes was performed with the neighbour-joining method and was calculated by the Kimura 2-parameter model with 2,000 bootstrap replicates.
The results of the B-ultrasound examination of the patient showed that the gallbladder was 97×44 mm in size, indicating that it was slightly enlarged. The capsule wall was smooth, the liver edge was smooth, and the echo in the parenchymal area was dense. Therefore, the initial diagnosis was fatty liver and acute cholecystitis. The routine blood test results showed that the percentages of monocytes, eosinophils, and basophils were 11.2%, 11.4%, and 1.5%, respectively, which were all higher than the standard values. The total protein and albumin levels in the patient’s serum were lower than the standard values of 64.2 g/L and 36.2 g/L, respectively. Accordingly, the preliminary diagnosis included a suspected parasitic infection and hypoalbuminemia.
The patient’s stool occult blood test was positive. The tapeworm eggs in the stool sample were oval in shape and contained oncospheres (
Fig. 1B). After 6 hr of intestinal deworming with medication, a milky white tapeworm was excreted from the patient. The worm body was thick, approximately 146 cm in length and with whitish-yellow in colour (
Fig. 1A); the widest part of the gravid proglottid of the worm body was approximately 10 mm, and the worm was intact with a visible scolex (
Fig. 1C). The scolex was rectangular in shape with 4 suckers and without hooklet and rostellum examined using light microscopy.
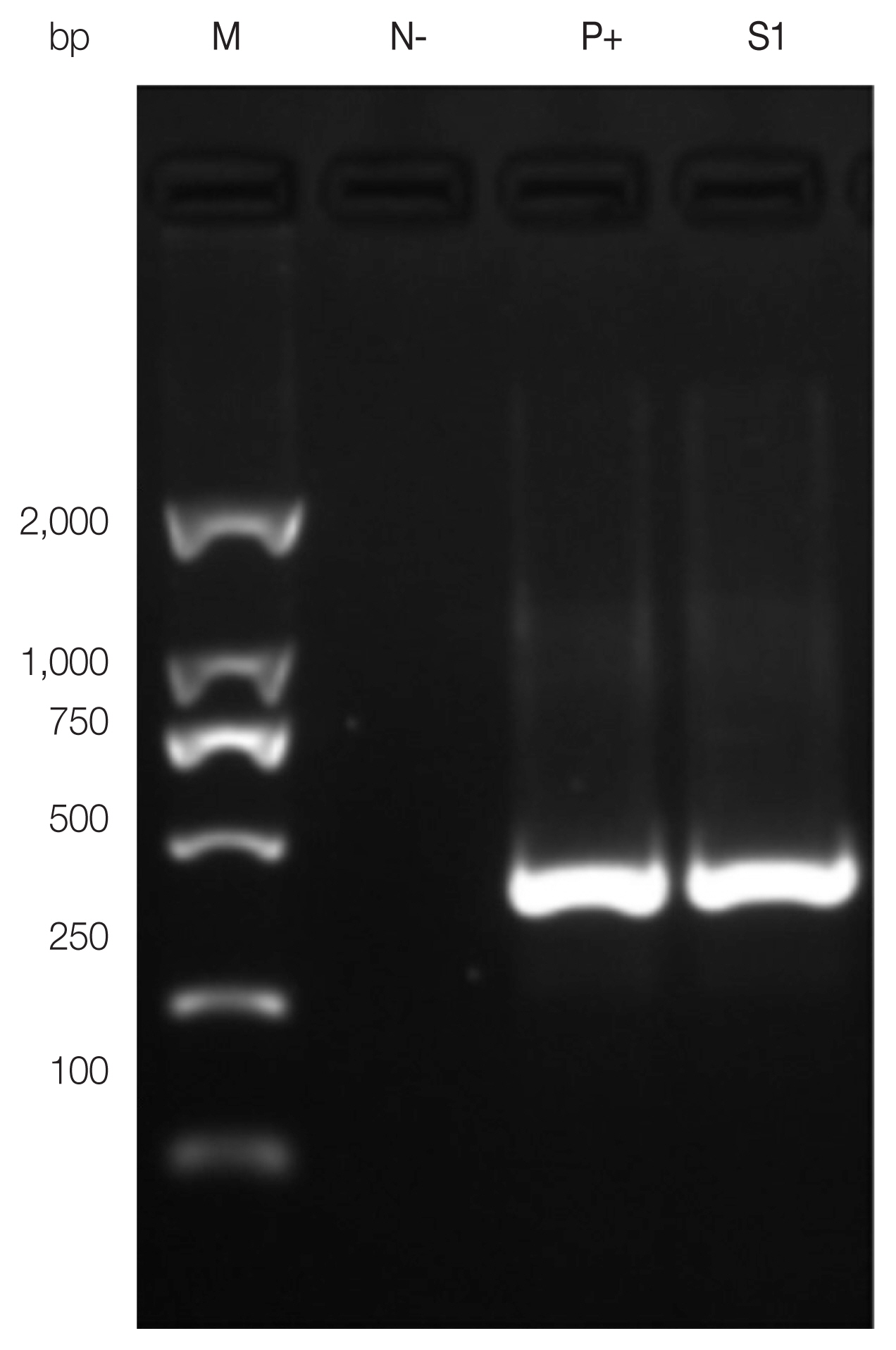
The 440-bp fragment of the
cox1 gene amplified by PCR was observed under UV light (
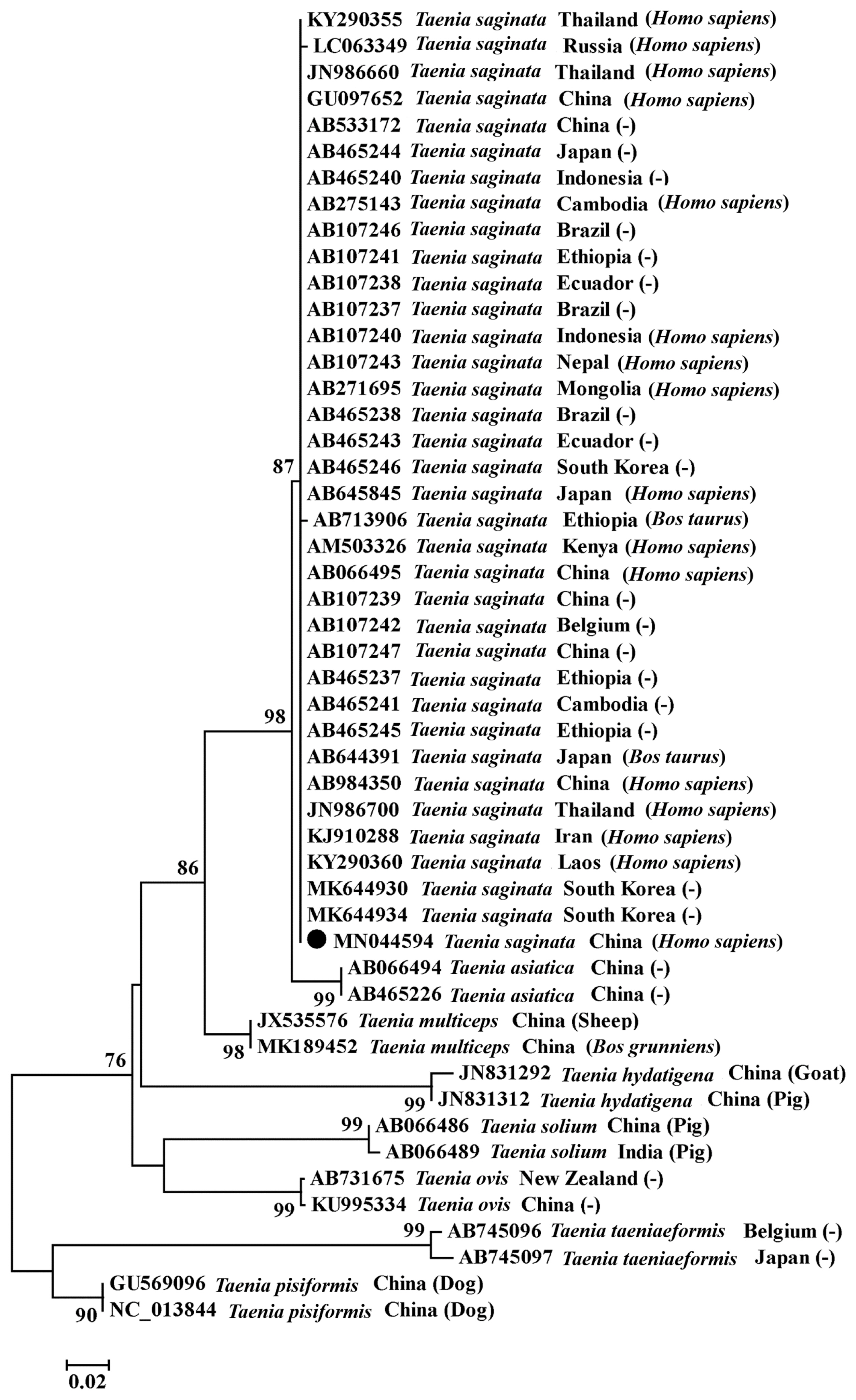
Fig. 2). A sequence alignment showed that the amplified
cox1 sequence of this case shared 100% identity with that of
T. saginata isolate in Iran (KJ910288) and 99.5–99.8% homology with
T. saginata (AB066495, AB984350, AB107239, and AB533172) from other regions in China (
Fig. 3). The patient was identified infected with
T. saginata.
As the public health situation has recently greatly improved and living habits are more hygienic, parasite infection was not considered in the initial diagnosis, so the patient was initially diagnosed with acute cholecystitis. For the following reasons, the patient reported a slight high fever and the development of pain in her upper abdomen, intermittent pain and discomfort under the xiphoid, radiating pain in the left back and vomiting or loss of appetite. After a medical examination, the pain was determined to be located in the right upper abdomen and under the xiphoid at 3 horizontal finger-lengths below the ribs, and there were no lumps in the abdomen. The gallbladder was positive for Murphy’s sign. The routine blood test results showed that the percentages of monocytes, eosinophils, and basophils were all higher than the standard values. This finding is in line with the characteristics of an elevated WBC count. The results of the B-ultrasound examination of the patient were basically consistent with the characteristics of acute cholecystitis. Therefore, the preliminary diagnosis was acute cholecystitis. Unexpectedly, it was truly a parasite infection. Next, we reviewed some information regarding this case. Actually, we ignored some clues: the patient was in close contact with dogs, yaks and sheep; she also had a habit of eating raw yakiniku and air-dried meat, which implied that the symptoms may be caused by parasite infection (existing infection source), although these are normal attributes of the lives of Tibetan herders. It was necessary to comprehensively consider the causes, and the necessary examinations and laboratory diagnosis should be performed. Based on this information, correct treatment measures could be adopted and implemented, especially for neglected and relatively rare parasitic infection cases.
Eventually, based on the molecular detection results, it was determined that the patient was infected with
T. saginata. It was reported that tapeworm
T. solium caused cholecystitis in an uncommon case of biliary taeniasis [
15]. In preliminary results of a survey of taeniasis /cysticercosis in the Ganze Tibetan prefecture regions, the prevalence of
T. saginata infection was 22.5%, with
T. saginata as the dominant species [
16].
T. saginata is especially prevalent in areas in which people eat raw or semiraw beef. Infection is closely related to local socioeconomic development, lifestyles and dietary cultural habits [
17]. To date, there has been no systematic investigation of
T. saginata cysticercus infection in Qinghai Province. However, according to quarantine data, there are only individual reports of yak cysticercosis in some regions. In 2005, cysticerci were found in the muscle tissue of yaks slaughtered in Qumalai County, Yushu prefecture [
18]. The above results suggest that bovine taeniasis does exist in the pastoral areas of Qinghai Province. In the future, it will be necessary to perform epidemiological investigations of the disease in humans and livestock. Tibetan herders migrate with the seasons to ensure the growth and development of their domestic animals on the Qinghai-Tibet Plateau, and favorable conditions for
T. saginata infection and transmission exist due to the traditional diets and lifestyles of the Tibetan populations in Qinghai Province.
The patient was treated with a traditional deworming method, the pumpkin seed-betel nut deworming method. A complete tapeworm body with a visible scolex was obtained, and tapeworm infection was confirmed, indicating that the traditional treatment method was very effective. Chinese scientists have explored the action modes and clinical administration methods of pumpkin seeds and betel nuts, which is still the main clinical regimen for the disease [
19]. Another study showed that pumpkin seed/areca combined treatment was safe and highly effective (89%) for human taeniasis [
20].
Because the morphology and size of tapeworm eggs are very similar, it is difficult to distinguish the species under light microscopy. Morphological characteristics such as the numbers of proglottids and uterine side branches and the scolex can be used to distinguish parasite species. However, T. saginata and T. asiatica have very similar morphological characteristics of the gravid proglottid and scolex. In addition, it is difficult to obtain adult worms from most people with tapeworm infections. Therefore, it is important to improve the detection and identification of tapeworms to ensure accurate parasite species identification.
At present, sequencing of the mitochondrial genome of tapeworms has been completed, and the overall difference in the whole mitochondrial genome sequence of
T. saginata and
T. asiatica was 4.6% [
21]. The mitochondrial genes
cox1,
cytb, and
nad1 and other genes can be used as markers for identification of tapeworms. In 2014,
cox1 gene of
T. saginata collected from a man was sequenced to compare
T. saginata homology to
T. asiatica and
T. solium [
22]. Its sequence homology was 94.7–94.9% and 87.8–88.0%, respectively. The present case (MN044594) shared 99.5–99.8% homology with sequences of
T. saginata from other regions in China and 100% identity with that of
T. saginata isolate in Iran (KJ910288).
Taeniasis cases are in Tibetan populations in Qinghai Province, but the epidemic status has not yet been determined. Prevention and control measures should be strengthened, and eating and hygiene habits should be improved among the Tibetan herders.
Notes
-
The authors have no conflicts of interest to declare.
ACKNOWLEDGMENTS
This study was carefully reviewed by the ethics committee of Qinghai Provincial People’s Hospital in accordance with relevant medical ethics regulations, because the study involved the isolation of T. saginata from a patient and was therefore subject to the principles of medical ethics. We obtained approval (Approval No. 2016-12) from the ethics committee, ensuring that the study met the relevant requirements of the ethics committee and maintained the rights of the patient. This study was focused only on T. saginata isolated from a patient and did not involve any personal privacy information (individual details, images or videos). Before T. saginata deworming, the patient was informed that the tapeworm would be further identified or used for scientific research. She knew this and agreed to the publication of biological information about the tapeworm.
We are grateful to the staff working in Qinghai Provincial People’s Hospital for their assistance with collecting the samples and data. This research was supported by the Basic Scientific Independent Research Project of Qinghai Academy of Animal Science and Veterinary Medicine (MKY-2019-10) and the Qinghai University Young and Middle Research Foundation Project (2018-QNY-1).
Fig. 1Strobila and egg of Taenia saginata. (A) Whole strobila with scolex (arrow), 146 cm long. (B) An egg obtained from feces. (C) Scolex of T. saginata.

Fig. 2Amplicons of cox1 gene PCR-amplified using Taenia universal primers. M: 2,000 bp DNA molecular marker. N−, negative control; P+, positive control; S1, present case.

Fig. 3A phylogenetic tree constructed based on the cox1 sequences of Taenia spp. using neighbour-joining method. ●, cox1 gene amplified in this study. (−), host information was not applicable.

References
- 1. Yang YM, Wu XL, Tian HC. Investigation report on human Taenia infection in China. China Tropical Medicine 2019;19:4-6. (in Chinese). https://doi.org/10.13604/j.cnki.46-1064/r.2019.01.02
- 2. Chen YH, Guo ZX. Distribution and control of zoonotic parasites in Qinghai Plateau. Chin J Parasitol Parasitic Dis 1994;12(suppl):85-88. (in Chinese). https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CJFD&dbname=CJFD9495&filename=ZJSB4S1.025&v=c%25mmd2FDkUYEFZCRpWu%25mmd2FsoEKMigclzOWnCNmUGk4GN2pH%25mmd2BY%25mmd2BupcbvTZysg%25mmd2FqBTS0mnifM
- 3. Xu LQ, Chen YD, Sun FH, Cai L, Fang YY, Wang LP, Liu X, Li LS, Feng Y, Li H. Coordinating Office of the National Survey on the Important Human Parasitic Diseases. A National Survey on Current Status of the Important Parasitic Diseases in Human Population. Chin J Parasitol Parasitic Dis 2005;23(suppl):332-340. (in Chinese). https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CJFD&dbname=CJFD2005&filename=ZJSB2005S1005&v=tOXrP21wUSrDbCHs6tajr8EhcVGyviXXAY4BK646s8GoRwu0B0YnensdftTNa2MO
- 4. World Health Organization. Generic Framework for Control, Elimination and Eradication of Neglected Tropical Diseases; Geneva, Switzerland: World Health Organization; 1-6 https://www.who.int/neglected_diseases/resources/WHO_HTM_NTD_2016.6/en/
- 5. Melkie G, Tewodros AE. Review on taeniasis and its zoonotic importance. Europ J Appl Sci 2015;7:182-191. https://doi.org/10.5829/idosi.ejas.2015.7.4.96169
- 6. Eichenberger RM, Thomas LF, Gabriël S, Bobić B, Devleesschauwer B, Robertson LJ, Saratsis A, Torgerson PR, Braae UC, Dermauw V, Dorny P. Epidemiology of Taenia saginata taeniosis/cysticercosis: a systematic review of the distribution in East, Southeast and South Asia. Parasite Vectors 2020;13:234. https://doi.org/10.1186/s13071-020-04095-1
- 7. Won EJ, Jung BK, Song H, Kim MS, Kim HS, Lee KH, Kim MJ, Shin MG, Shin JH, Suh SP, Hong SJ, Sohn WM, Htoon TT, Tin HH, Chai JY. Molecular diagnosis of Taenia saginata tapeworm infection in 2 schoolchildren, Myanmar. Emerg Infect Dis 2018;24:1156-1158. https://doi.org/10.3201/eid2406.180217
- 8. Li T, Chen X, Yanagida T, Wang H, Long C, Sako Y, Okamoto M, Wu Y, Giraudoux P, Raoul F, Nkouawa A, Nakao M, Craig PS, Ito A. Detection of human taeniases in Tibetan endemic areas, China. Parasitology 2013;140:1602-1607. https://doi.org/10.1017/S003118201300111X
- 9. Zhao HL, Ma SM, Cao DP. The analysis of 128 cases of taeniasis and cysticercosis patients in Qinghai Province. Acta Parasitologica et Medica Entomologica Sinica 2007;14:56-58. (in Chinese). https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CJFD&dbname=CJFD2007&filename=JSCY200701011&v=UX9BxOQmjJw3yQ7CP00khXd9BsXTJbQYpwc%25mmd2FOZeEyVyVtEQWrjaLxioh8RPvYTaI
- 10. Jeon HK, Eom KS. Molecular approaches to Taenia asiatica. Korean J Parasitol 2013;51:1-8. https://doi.org/10.3347/kjp.2013.51.1.1
- 11. Ale A, Victor B, Praet N, Gabriël S, Speybroeck N, Dorny P, Devleesschauwer B. Epidemiology and genetic diversity of Taenia asiatica: a systematic review. Parasite Vectors 2014;7:45. https://doi.org/10.1186/1756-3305-7-45
- 12. Song SM, Yun HS, VanBik D, Chang HH, Lee SA, Kim SW, Ryoo N, Eun DY, Lee NY, Goo YK, Hong Y, Ock M, Cha HJ, Chung DI. Ten cases of Taenia saginata infection confirmed by analysis of the internal transcribed spacer 1 rDNA region in the Republic of Korea. Korean J Parasitol 2019;57:417-422. https://doi.org/10.3347/kjp.2019.57.4.417
- 13. Silva CV, Costa-Cruz JM. A glance at Taenia Saginata infection, diagnosis, vaccine, biological control and treatment. Infect Disord Drug Targets 2010;10:313-321. https://doi.org/10.2174/187152610793180894
- 14. Guo ZH, Kubo M, Kudo M, Nibe K, Horii Y, Nonaka N. Growth and genotypes of Echinococcus granulosus found in cattle imported from Australia and fattened in Japan. Parasitol Int 2011;60:498-502. https://doi.org/10.1016/j.parint.2011.09.002
- 15. Yu HJ, Ahn CS, Lim S, Kim JG, Kim MS, Chae SW, Yeom JS, Joo EJ, Sohn WM, Kwon MJ. Biliary Taeniasis with Cholecystitis: an unusual case of Taenia solium infection with a literature review. Am J Trop Med Hyg 2019;100:135-139. https://doi.org/10.4269/ajtmh.18-0633
- 16. Li T, Craig PS, Ito A, Chen X, Qiu D, Qiu J, Sato MO, Wandra T, Bradshaw H, Li L, Yang Y, Wang Q. Taeniasis/cysticercosis in a Tibetan population in Sichuan Province, China. Acta Trop 2006;100:223-231. https://doi.org/10.1016/j.actatropica.2006.11.003
- 17. Huarui DZ. On the food culture of the Anduo Tibetans. J Tibet Univ Nationalities 2014;35:30-35. (in Chinese). https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CJFD&dbname=CJFD2014&filename=XZMZ201403007&v=gnpLZDf11HHH6cK%25mmd2BBdT2DnyDl3iWIbAAV4v2nqqZtyTsXMJpuRnV78JVtd1q1Psu
- 18. Yang GX. A case of yak cysticercosis. Qinghai J Anim Sci Vet Med 2005;1:39. (in Chinese). https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CJFD&dbname=CJFD2005&filename=QXSZ200501048&v=B9D3cuM3GmFTf2hFWAfeIm%25mmd2F1lrk8m1%25mmd2FFIdljswcpWvdYGVBd4zNwmD3m1P9ySoFW
- 19. Zhang H, Liu C, Zheng Q. Development and application of anthelminthic drugs in China. Acta Trop 2019;200:105181. https://doi.org/10.1016/j.actatropica.2019.105181
- 20. Li T, Ito A, Chen X, Long C, Okamoto M, Raoul F, Giraudoux P, Yanagida T, Nakao M, Sako Y, Xiao N, Craig PS. Usefulness of pumpkin seeds combined with areca nut extract in community-based treatment of human taeniasis in northwest Sichuan Province, China. Acta Trop 2012;124:152-157. https://doi.org/10.1016/j.actatropica.2012.08.002
- 21. Jeon HK, Kim KH, Eom KS. Complete sequence of the mitochondrial genome of Taenia saginata: comparison with T. solium and T. asiatica. Parasitol Int 2007;56:243-246. https://doi.org/10.1016/j.parint.2007.04.001
- 22. Cho J, Jung BK, Lim H, Kim MJ, Yooyen T, Lee D, Eom KS, Shin EH, Chai JY. Four cases of Taenia saginata, infection with an analysis of cox1 gene. Korean J Parasitol 2014;52:79-83. https://doi.org/10.3347/kjp.2014.52.1.79