Abstract
Global efforts to identify groups at high risk for schistosomiasis have mainly concentrated on identifying their geographical distribution. Investigations on the socioeconomic characteristics of high-risk groups are relatively scarce. This study aimed to explore the associations between schistosomiasis among students and their parents’ occupations. A nationwide cross-sectional survey was conducted targeting 105,167 students in 1,772 primary schools across Sudan in 2017. From these students, 100,726 urine and 96,634 stool samples were collected to test for Schistosoma haematobium and S. mansoni infection. A multi-level mixed effect analysis was used with age and sex as fixed factors, and school as a random factor. The odd ratios (ORs) of practicing open defecation among farmers’ children were almost 5 times higher than their counterparts whose parents were government officials (OR=4.97, 95% confidence intervals (CIs): 4.57–5.42, P<0.001). The ORs of contacting water bodies for watering livestock among farmers’ children were more than 4 times higher than those of children whose parents were government officials (OR=4.59, 95% CIs: 4.02–5.24, P<0.001). This study shows that schistosomiasis represents a disease of poverty and that farmers’ children constituted a high-risk group.
-
Key words: Schistosomiasis, parents’ occupation, poverty, water, sanitation, Sudan
The neglected tropical diseases (NTDs) are recognized as diseases of poverty since they are concentrated among marginalized people in hard-to-reach areas in low-income countries [
1–
4]. Schistosomiasis is a public health concern in 51 endemic countries [
5]. Of the 237 million people who required preventive chemotherapy in 2019, fewer than 50% were treated [
5]. In Africa, more than 112 million and 54 million individuals were reported to have urogenital and intestinal schistosomiasis, respectively [
6,
7].
The World Health Organization (WHO) aims to achieve at least 75% coverage of preventive chemotherapy in every country where schistosomiasis is endemic [
8,
9] The coverage of preventive chemotherapy against key NTDs has been suggested as a litmus test for assessing progress towards universal health coverage, the ultimate target of Sustainable Development Goal 3, because the characteristics of these diseases represent the symptoms of poverty [
10,
11]. According to the recent reports, fewer than 50% of the people at risk of schistosomiasis received preventive chemotherapy in sub-Saharan Africa. The global health strategy against schistosomiasis highlights an integrated approach embracing water and sanitation improvements [
1,
9]. The global health community has emphasized the importance of identifying groups at high risk for infection transmission [
3,
9,
12].
Considerable achievements have been made in reducing the prevalence of schistosomiasis in Sudan over the past few decades [
13,
14]. The nationwide prevalence dropped below 6% in 2017 [
13]. These substantial gains were achieved through the concerted efforts of the Federal Ministry of Health, Sudan (FMOH) and development partners, including WHO, the United Nations Children’s Fund, the Schistosomiasis Control Initiative, and the Korea International Cooperation Agency (KOICA) [
13]. FMOH is now developing a new strategy to transition from control towards elimination of schistosomiasis [
15]. Understanding the characteristics of high-risk groups is critical for eliminating the disease by reducing and maintaining the prevalence of heavy infections below 1% [
9]. In this regard, KOICA supported a nationwide mapping project to identify high-risk groups in terms of geographical distribution, the results of which have been published elsewhere [
13]. However, it is necessary to understand other characteristics of high-risk groups.
This study explored the associations between schistosomiasis among students and their parents’ occupations. Understanding children’s schistosomiasis prevalence, as well as their risk factors and risk behaviors according to their parents’ occupation, is critical to help FMOH to make informed decisions for formulating a more tailored intervention strategy.
This is an additional study following a nationwide geographical analysis based on a publicly available dataset [
16]. This study was also conducted on the basis of a request made by FMOH to provide clear information on the schistosomiasis distribution among children according to their parents’ occupation while the authors participated in the NTD elimination and network strengthening project.
Ethical approval was obtained from both FMOH ethical committee (FMOH/DGP/RD/TC/2016; January 15, 2017) and the Korea Association of Health Promotion (130,750–20,164-HR-020; May 16, 2016). Informed consent was obtained in written form. The basic characteristics of the students who participated in the survey are presented in
Table 1. A nationwide cross-sectional survey was conducted targeting 105,167 students in 1,771 primary schools across 183 districts of 18 states in Sudan. The study protocol was published prior to the first nationwide large-scale survey conducted throughout Sudan [
17]. Sampling was done using the probability-proportional-to-size method. Laboratory specialists and data collectors were temporarily hired and trained by the project team from the existing workforce of hospitals, the State Ministry of Health, or private institutions. The Kato-Katz and centrifugation methods were used to examine urogenital and intestinal schistosomiasis, respectively.
In total, 105,167 students were interviewed to identify their demographic characteristics, water and sanitation at the household and school level, and behavior relating to regular contact with water bodies such as rivers, irrigation canals, and ponds. From these respondents, 100,726 urine and 96,634 stool samples were collected to test for Schistosoma haematobium and S. mansoni infection. The sex ratio of children ranged from 1.0 in the White Nile, Al Qadarif, and Northern States to 1.5 in Khartoum and North Kordofan States. The mean age of the 105,167 participants was 10.9 years (±2.3 years).
This study first investigated associations between water and sanitation status at students’ households and their parents’ occupation (categorized into farmers, teachers, government officials, small-scale businessmen and traders, and others). We excluded others and non-responders. The associations between students’ risk behaviors and their parents’ occupation were investigated. We also evaluated the extent of associations between students’ schistosomiasis infection and their parents’ occupation. In a supplementary analysis, associations between water and sanitation at the school level and parents’ occupations were explored with the expectation of deriving meaningful insights for schistosomiasis-related government policies. For the statistical analysis, a multi-level mixed effect analysis was used with age and sex as fixed factors, and school as a random factor. STATA 16 (StataCorp, College Station, Texas, USA) was used for the analysis.
Data on the prevalence of
S. haematobium and
S. mansoni have previously been published [
13]. Basic characteristics of the survey participants were described in
Supplementary Table S1. The geographic distribution of prevalence is not the topic of this study. However, we analyzed schistosomiasis prevalence regardless of the type (
S. haematobium or
S. mansoni), co-infection (
S. haematobium and
S. mansoni), and co-infection with soil-transmitted helminthiasis (STH) to explain the underlying context of the study area, all of which have not been reported in previous study (
Table 1) [
13]. Based on the analysis of the nationwide data, co-infection (either schistosomiasis co-infection or co-infection of schistosomiasis and STH) was not a public health concern (
Table 1). This might be largely attributable to the low prevalence of
S. mansoni (0.06%) and STH (0.1%) [
13]. Accordingly, STH and co-infections were not considered in this study.
Water and sanitation status at household and school level according to parents’ occupation is described in
Table 2. Farmers’ children had the worst status for all variables, including the lowest proportions of those with improved water or sanitation at the household level and the highest proportion of those who practiced open defecation. Of note, the same held true at the school level; a possible explanation for this is that children of parents with certain occupations, particularly farmers, are clustered and thus some schools may be mainly composed of farmers’ children. The odds of practicing open defecation among farmers’ children were almost 5 times higher (odds ratio [OR]=4.97, 95% confidence intervals [CIs]: 4.57–5.42,
P<0.001) than their counterparts whose parents were government officials. Farmers’ children had lower odds of having improved sanitation in their households than their counterparts (OR=0.28, 95% CIs: 0.26–0.29,
P<0.001) (
Table 2).
Table 3 shows children’s risk behaviors according to their parents’ occupation. The proportion of respondents who had regular contact with water bodies (at least 3 times per week) was highest among the children of farmers for all indicators under the category of water contact. For instance, their odds of contacting water bodies for fetching water were more than 3 times higher than those of children whose parents were government officials (OR=3.17, 95% CIs: 2.93–3.44,
P<0.001). Furthermore, their odds of contacting water bodies for watering livestock were more than 4 times higher than those of children whose parents were government officials (OR=4.59, 95% CIs: 4.02–5.24,
P<0.001).
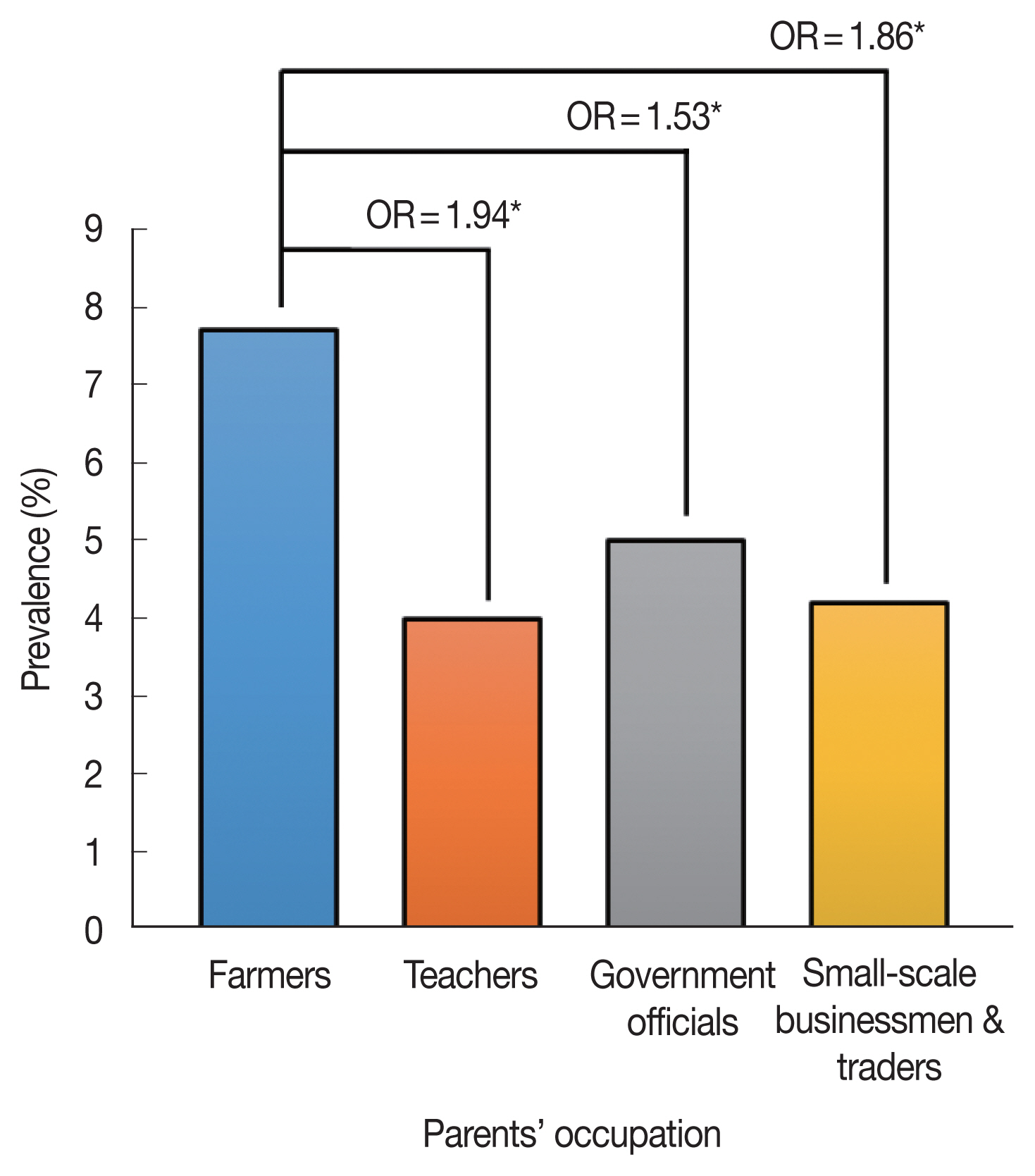
Fig. 1 shows the schistosomiasis prevalence of students according to their parents’ occupation. The children of farmers were more likely to be infected with schistosomes than any other group. The odds of farmers’ children having schistosomiasis were 53% higher than those of children whose parents were government officials (OR=1.53, 95% CIs: 1.39–1.69,
P< 0.001). Considering the low coverage of improved water and sanitation, farmers can be categorized as the poorest group analyzed in this study based on the definition of poverty [
11]. It is worth noting that the poorest group encountered the worst conditions even at the school level.
The findings in this study are consistent with the argument that irrigation canals are a key factor contributing to the high prevalence of schistosomiasis, particularly among farmers [
18–
22]. Many farmers rely on irrigation canals in Sudan, and snails, an intermediate host, reside in these canals [
18–
22]. This study demonstrated that farmers’ children were at high risk. Global efforts to identify groups at high risk for schistosomiasis have mainly concentrated on identifying their geographical distribution. Studies investigating the socioeconomic characteristics of high-risk groups are relatively scarce. This study shows that schistosomiasis is clearly a disease of poverty. The next step is to formulate a more tailored strategy targeting high-risk groups. This evidence can also be used to advocate for improving water and sanitation facilities at large-scale sugar cane farms covering substantial areas of land across Sudan. Sugar cane farmers are victims of schistosomiasis infection as a part of their routine occupational activities [
20].
Notes
-
The authors have no conflicts of interest.
Supplementary Information
ACKNOWLEDGMENTS
The authors thank the project team members for their efforts and contributions to controlling NTDs in Sudan. The authors extend their appreciation to community members, the Ministries of Heath of 18 states, and the Federal Ministry of Health, Sudan. Special thanks go to Mr. Hoo-Gn Jeoung, Director, Health Examination Managing Bureau, Korea Association of Health Promotion. The project was funded by KOICA (P-2015-00145). The authors also extend their appreciation to KOICA.
Fig. 1Schistosomiasis prevalence according to parents’ occupation. Odds ratio (ORs) adjusted for age and sex were estimated by setting teachers, government officials, small-scale businessmen, and traders as the reference group, respectively. *P<0.001.

Table 1Prevalence of schistosomiases and co-infections
Table 1
|
State |
No. of examined |
Schistosomiasis (%) (S. mansoni or S. haematobium) |
Co-infection (%) (S. mansoni and S. haematobium) |
Co-infection (%) of schistosomiasis and soil-transmitted helminthiasis |
|
Al Jazirah |
6,358 |
2.8 |
0.0 |
0.0 |
|
Al Qadarif |
6,059 |
6.2 |
0.2 |
0.0 |
|
Blue Nile |
3,950 |
2.6 |
0.2 |
0.1 |
|
Central Darfur |
4,441 |
3.4 |
0.0 |
0.0 |
|
East Darfur |
4,709 |
17.0 |
0.2 |
0.1 |
|
Kassala |
6,466 |
2.6 |
0.0 |
0.0 |
|
Khartoum |
3,596 |
5.6 |
0.1 |
0.0 |
|
North Darfur |
6,799 |
1.4 |
0.0 |
0.0 |
|
North Kordofan |
3,731 |
2.6 |
0.0 |
0.0 |
|
Northern |
3,859 |
0.4 |
0.0 |
0.0 |
|
Red Sea |
3,723 |
1.4 |
0.0 |
0.1 |
|
River Nile |
3,342 |
0.1 |
0.0 |
0.0 |
|
Sennar |
14,712 |
15.1 |
0.0 |
0.0 |
|
South Darfur |
9,477 |
5.0 |
0.0 |
0.0 |
|
South Kordofan |
3,068 |
1.1 |
0.1 |
0.1 |
|
West Darfur |
4,378 |
1.6 |
0.1 |
0.1 |
|
West Kordofan |
10,685 |
3.9 |
0.0 |
0.0 |
|
White Nile |
7,563 |
5.1 |
0.0 |
0.0 |
|
Total |
106,916 |
5.5 |
0.0 |
0.0 |
Table 2Water and sanitation status of children’s households and school according to their parents’ occupation
Table 2
|
Household level |
School level |
|
|
|
Improved latrine1
|
Open defecation |
Improved water2
|
Improved latrine |
|
Farmers (n=36,551) |
6.80% |
25.10% |
81.00% |
38.80% |
|
|
Odds ratio3
|
0.15 |
4.97 |
0.29 |
0.41 |
|
|
95% confidence interval |
0.14–0.16 |
4.57–5.42 |
0.26–0.33 |
0.39–0.43 |
|
|
P-value |
<0.001 |
<0.001 |
<0.001 |
<0.001 |
|
|
Odds ratio3
|
0.28 |
2.03 |
0.53 |
0.61 |
|
|
95% confidence interval |
0.26–0.29 |
1.96–2.10 |
0.50–0.55 |
0.60–0.63 |
|
|
P-value |
<0.001 |
<0.001 |
<0.001 |
<0.001 |
|
|
Teachers (n=2,408) |
27.8% (670) |
7.6% (184) |
91.3% (2,199) |
50.2% (1,209) |
|
|
Government officials (n=9,653) |
34.0% (3,278) |
6.4% (613) |
93.3% (9,011) |
60.4% (5,831) |
|
|
Small-scale businessmen/traders (n=41,122) |
18.2% (7,504) |
16.5% (6,772) |
88.6% (36,176) |
48.5% (19,961) |
Table 3Water contact behaviors of children according to their parents’ occupation
Table 3
|
Overall water contact2
|
Fetching water |
Bathing |
Washing clothes |
Watering livestock |
Swimming |
|
Farmers (n=36,551) |
51.00% |
21.10% |
18.70% |
11.30% |
10.60% |
20.50% |
|
Odds ratio1
|
2.11 |
3.17 |
2.00 |
1.96 |
4.59 |
1.28 |
|
95% confidence interval |
2.02–2.22 |
2.93–3.44 |
1.86–2.15 |
1.79–2.14 |
4.02–5.24 |
1.20–1.36 |
|
P-value |
<0.001 |
<0.001 |
<0.001 |
<0.001 |
<0.001 |
<0.001 |
|
Teachers (n=2,408) |
31.90% |
9.10% |
10.40% |
5.50% |
3.40% |
13.50% |
|
Government officials (n=9,653) |
32.00% |
7.70% |
10.10% |
5.90% |
2.50% |
16.50% |
|
Small-scale businessmen/traders (n=41,122) |
33.40% |
10.60% |
10.90% |
5.40% |
3.10% |
14.80% |
References
- 1. World Health Organization. Ending the neglect to attain the Sustainable Development Goals: A Global Strategy on Water, Sanitation and Hygiene to Combat Neglected Tropical Diseases 2021–2030. World Health Organization; Geneva, Switzerland. 2021, https://www.who.int/publications/i/item/9789240022782
- 2. World Health Organization. Ninth report of the Strategic and Technical Advisory Group for Neglected Tropical Diseases (STAG-NTDs). World Health Organization; Geneva, Switzerland. 2021, https://www.who.int/publications/m/item/ninth-report-of-the-strategic-and-technical-advisory-group-for-neglected-tropical-diseases-(stag-ntds)
- 3. Casulli A. New global targets for NTDs in the WHO roadmap 2021–2030. PLoS Negl Trop Dis 2021;15:e0009373. https://www.doi.org/10.1371/journal.pntd.0009373
- 4. Engels D, Zhou XN. Neglected tropical diseases: an effective global response to local poverty-related disease priorities. Infect Dis Poverty 2020;9:10. http://www.doi.org/10.1186/s40249-020-0630-9
- 5. World Health Organization. Schistosomiasis: Key Facts [Internet]. Geneva. World Health Organization; 2022, Available from: https://www.who.int/news-room/fact-sheets/detail/schistosomiasis
- 6. World Health Organization. Current estimated total number of individuals with morbidity and mortality due to Schistosomiasis haematobium and S. mansoni infection in Sub-Saharan Africa. Schistosomiasis; 2017. [Internet]Geneva: World Health Organization; 2021. Available from: https://www.who.int/teams/control-of-neglected-tropical-diseases/schistosomiasis/epidemiology#:~:text=A%20review%20of%20disease%20burden,schistosomiasis%20in%20sub%2DSaharan%20Africa
- 7. GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018;392:1789-1858. https://www.doi.org/10.1016/S0140-6736(18)32279-7
- 8. World Health Organization. Elimination of Schistosomiasis: World Health Assembly Resolution WHA 65.21 [Internet]; Available from: www.who.int/neglected_diseases/mediacentre/WHA_65.21_Eng.pdf
- 9. Tchuem Tchuenté LA, Rollinson D, Stothard JR, Molyneux D. Moving from control to elimination of schistosomiasis in sub-Saharan Africa: time to change and adapt strategies. Infect Dis Poverty 2017;6:42. https://www.doi.org/10.1186/s40249-017-0256-8
- 10. Fitzpatrick C, Bangert M, Mbabazi PS, Mikhailov A, Zouré H, Polo Rebollo M, Robalo Correia E, Silva M, Biswas G. Monitoring equity in universal health coverage with essential services for neglected tropical diseases: an analysis of data reported for five diseases in 123 countries over 9 years. Lancet Glob Health 2018;6:e980-988. https://www.doi.org/10.1016/S2214-109X(18)30307-3
- 11. United Nations. The Sustainable Development Goals Report 2020; United Nations; New York, USA: 2020. https://unstats.un.org/sdgs/report/2020/
- 12. Bizimana P, Ortu G, Van Geertruyden JP, Nsabiyumva F, Nkeshimana A, Muhimpundu E, Polman K. Integration of schistosomiasis control activities within the primary health care system: a critical review. Parasit Vectors 2019;12:393. https://www.doi.org/10.1186/s13071-019-3652-z
- 13. Cha S, Elhag MS, Lee YH, Cho DS, Ismail HAHA, Hong ST. Epidemiological findings and policy implications from the nationwide schistosomiasis and intestinal helminthiasis survey in Sudan. Parasit Vectors 2019;12:429. https://www.doi.org/10.1186/s13071-019-3689-z
- 14. Charani E, Cunnington AJ, Yousif AHA, Seed Ahmed M, Ahmed AEM, Babiker S, Badri S, Buytaert W, Crawford MA, Elbashir MI, Elhag K, Elsiddig KE, Hakim N, Johnson MR, Miras AD, Swar MO, Templeton MR, Taylor-Robinson SD. In transition: current health challenges and priorities in Sudan. BMJ Glob Health 2019;4:e001723. https://www.doi.org/10.1136/bmjgh-2019-001723
- 15. Federal Ministry of Health. A Neglected Tropical Diseases Strategy. Federal Ministry of Health; Khartum, Sudan. 2021.
- 16. Elhag MS, Jin Y, Amin MA, Ismail HAHA, Hong ST, Jang HI, Doh Y, Cha S. Cost and logistics implications of a nationwide survey of schistosomiasis and other intestinal helminthiases in Sudan: key activities and cost components. PLoS One 2020;15:e0226586. https://www.doi.org/10.1371/journal.pone.0226586
- 17. Cha S, Hong ST, Lee YH, Lee KH, Cho DS, Lee J, Chai JY, Elhag MS, Khaled SGA, Elnimeiri MKM, Siddig NAA, Abdelrazig H, Awadelkareem S, Elshafie ATE, Ismail HAHA, Amin M. Nationwide cross-sectional survey of schistosomiasis and soil-transmitted helminthiasis in Sudan: study protocol. BMC Public Health 2017;17:703. https://www.doi.org/10.1186/s12889-017-4719-4
- 18. Amin M, Abubaker H. Control fo schistosomiasis in the Gezira irrigation scheme, Sudan. J Biosoc Sci 2017;49:83-98. https://www.doi.org/10.1017/S0021932016000079
- 19. Eltayeb NM, Mukhtar MM, Mohamed AB. Epidemiology of schistosomiasis in Gezira area Central Sudan and analysis of cytokine profiles. Asian Pac J Trop Med 2013;6:119-125. https://www.doi.org/10.1016/S1995-7645(13)60006-1
- 20. Fenwick A, Cheesmond AK, Amin MA. The role of field irrigation canals in the transmission of Schistosoma mansoni in the Gezira Scheme, Sudan. Bull World Health Organ 1981;59:777-786. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2396103/pdf/bullwho00422-0129.pdf
- 21. Hilali AH, Madsen H, Daffalla AA, Wassila M, Christensen NO. Infection and transmission pattern of Schistosoma mansoni in the Managil irrigation scheme, Sudan. Ann Trop Med Parasitol 1995;89:279-286. https://www.doi.org/10.1080/00034983.1995.11812953
- 22. Mohammed EH, Eltayeb M, Ibrahim H. Haematological and biochemical morbidity of schistosoma haematobium in school children in Sudan. Sultan Qaboos Univ Med J 2006;6:59-64. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3074920/pdf/squmj-06-59.pdf
 , Seungman Cha2,3,*
, Seungman Cha2,3,* , Youngjin Kim2, Hamdan Mustafa Hamdan4, Mousab Siddig Elhag4, Hassan Ahmed Hassan Ahmed Ismail4
, Youngjin Kim2, Hamdan Mustafa Hamdan4, Mousab Siddig Elhag4, Hassan Ahmed Hassan Ahmed Ismail4 , Keon Hoon Lee5, Sung-Tae Hong6
, Keon Hoon Lee5, Sung-Tae Hong6