Abstract
Inflammatory bowel disease (IBD) is a chronic and recurrent illness of the gastrointestinal tract. Treatment of IBD traditionally involves the use of aminosalicylic acid and steroids, while these drugs has been associated with untoward effects and refractoriness. The absence of effective treatment regimen against IBD has led to the exploration of new targets. Parasites are promising as an alternative therapy for IBD. Recent studies have highlighted the use of parasite-derived substances, such as excretory secretory products, extracellular vesicles (EVs), and exosomes, for the treatment of IBD. In this report, we examined whether EVs secreted by Giardia lamblia could prevent colitis in a mouse model. G. lamblia EVs (GlEVs) were prepared from in vitro cultures of Giardia trophozoites. Clinical signs, microscopic colon tissue inflammation, and cytokine expression levels were detected to assess the effect of GlEV treatment on dextran sulfate sodium (DSS)-induced experimental murine colitis. The administration of GlEVs prior to DSS challenge reduced the expression levels of pro-inflammatory cytokines, including tumor necrosis factor alpha, interleukin 1 beta, and interferon gamma. Our results indicate that GlEV can exert preventive effects and possess therapeutic properties against DSS-induced colitis.
-
Key words: Giardia lamblia, inflammatory bowel disease, extracellular vesicle, dextran sulfate sodium
INTRODUCTION
Inflammatory bowel disease (IBD), which is characterized by the development of idiopathic chronic inflammation in the gastrointestinal tract, is typically represented by its 2 common forms, Crohn’s disease and ulcerative colitis. The symptoms of IBD usually include diarrhea, weight loss, and rectal bleeding between the ages of 15 and 35 years [
1]. Approximately 6–8 million cases of IBD have occurred globally in 2017. There are many cases in developed countries such as the USA and Europe; however, IBD incidence has recently been increased in other countries, including several Asian countries [
2]. Although the pathogenesis and etiology of IBD remain elusive, several studies have indicated that IBD is not only caused by defects in the immune function, genetic, and environmental factors, but also by dysbiosis of the intestinal flora [
3]. Several pharmacological drugs, such as aminosalicylate, steroids, immune modifiers, and antibiotics, are available for IBD treatment. However, the use of such pharmacological drugs is limited by side effects and refractoriness. More seriously, there is no curative treatment for IBD [
4,
5]. Several efforts have recently been undertaken to develop chemotherapeutics with fewer side effects [
6,
7].
Immune responses elicited by parasites have been suggested for the prevention and treatment of autoimmune disorders [
8,
9]. Some studies highlight a correlation between infections with various parasites and the development of immune disorders in humans and experimental models. In a clinical trial,
Trichuris suis ova reduced the symptoms and pathology of Crohn’s disease and ulcerative colitis in humans [
10].
Schistosoma mansoni and
Heligmosomoides polygyrus ameliorate inflammation in a chemical-induced colitis mouse model [
11,
12]. Certain parasite-derived substances, such as excretory secretory products (ESPs), extracellular vesicles (EVs), and exosomes, also exhibit immunoregulatory properties that drive intercellular interactions and parasite–host communication in inflammatory diseases.
Fasciola hepatica EVs exert a preventive effect on acute colitis in an experimental model [
13]. The schistosome enzymatic protein P28 glutathione-S-transferase (P28GST) reduces intestinal inflammation in a mouse colitis through increased Th2 responses [
14,
15]. These findings highlight the utility of parasite-derived molecules as drugs and more need for the identification of parasite-derived products.
Giardia lamblia is a gastrointestinal protozoa which releases ESPs and EVs that can modulate the host immune system [
16,
17]. Although previous reports indicated that
Giardia-derived molecules induce cytokine production and function as chemoattractants for neutrophils in vitro and in vivo [
18,
19], the potential use of
G. lamblia-derived EVs (GlEVs) as an immune modulator against IBD has remained unexplored. In this study, we evaluated GlEVs as a novel preventive agent against IBD by examining its effect on colitis using a dextran sulfate sodium (DSS)-induced colitis mouse model. The effect of GlEVs against DSS-induced colitis was investigated by determining disease activity index (DAI) and histological observation of tissue samples.
MATERIALS AND METHODS
Giardia lamblia culture and EV isolation
GlEVs were prepared as previously described [
16].
G. lamblia trophozoites (ATCC 30957) were cultured for 72 h in 10 ml tubes filled with TYI-33 medium at 37°C under microaerophilic conditions. Confluent
Giardia cultures were used to isolate EVs. First, TYI-33 medium was decanted from each tube following washing in phosphate buffered saline (PBS) (GenDEPOT, Barker, Texas, USA). Culture tubes were refilled with Dulbecco’s Modified Eagle medium (Gibco, Grand Island, New York, USA) for 6 h. At the end of incubation, culture tubes were place on ice for 10 min and centrifuged at 1,000×g for 10 min to remove debris. Supernatants were filtered and concentrated using a 100K Ultrafiltration membrane (Merck Milipore, Burlington, Massachusetts, USA) and centrifuged. The ExoQuick TC Kit (SBI System Bioscience, Menlo Park, California, USA) was mixed with concentrated medium (1:5) and incubated overnight at 4°C. GlEVs were resuspended in PBS buffer. The particle size and number were measured using Nanoparticle tracking analysis (NTA). NTA was performed on the NanoSight LM10 (Malvern Panalytical, Malvern, UK) and NTA software, Version 2.3 Build 2.3.5.0033.-Beta 7. GlEV were diluted at 1:10,000 in PBS. Sample were measured in triplicate at 25°C.
Five-week-old male C57BL/6 mice were purchased from OrientBio (Seongnam, Korea) and housed in a specific pathogen-free facility. All animal experiments were performed according to the guidelines for ethical conduct in the care and use of animals and were approved by the Centers for Disease Control and Prevention Institutional Animal Care and Use Committee (KCDC-IACUC; approval number: KCDC-018-14-2A). The mice were randomly divided into 3 groups, 5 in each group: Control group, DSS group, and DSS with GlEV group.
Induction of acute DSS-induced colitis and treatment with GIEVs
Acute colitis was induced via the administration of DSS (MP biomedicals, California, USA) in drinking water (3%, wt/vol) for 7 days, followed by normal drinking water. The control group received regular drinking water. Treatments with GlEVs were conducted via intraperitoneal (i.p.) injection with 10 μg per mouse 3 times (day −30, −23, and −16), as modified previously [
13].
The clinical signs of colitis were evaluated based on the DAI using previously described methods [
20]. Briefly, mice were weighed daily, and clinical signs were recorded. Each score was determined as follows: (a) weight loss (0=no loss, 1=1–5% weight loss, 2=5–10% loss, 3=10–20% loss, 4=more than 20% loss); (b) stool consistency (0=normal, 2=loose stools, 4=diarrhea); (c) bleeding (0=no bleeding, 2=microscopic bleeding, 4=gross bleeding).
The colon tissue was fixed in 4% formaldehyde, embedded in paraffin, and cut into 4 μm thick pieces with a microtome. The sections were stained with haematoxylin and eosin (H&E). Histological analysis was performed on transverse sections of H&E-stained tissues. The following parameters were used for calculations: (a) inflammatory cell infiltrate (0=none, 1= minimal severity at <10%, 2=severity at 10–25%, 3=severe at 26–50%); (b) degree of epithelial changes (0=none, 1=goblet cell loss at <25%, 2=goblet cell loss at 25–35%, 3=goblet cell loss at 36–50%); (c) change of mucosal (0=no changes, 1=villous blunting, 2=irregular crypts, 3=crypt loss). To quantitate the inflammatory cell infiltration, sections were stained with Lymphocyte antigen 6 complex locus G6D (Ly6G) monoclonal antibodies (ab25377, 1:400) (Abcam, Cambridge, UK), and evaluated the immunoreactivity with scores ranged from 0 to 3 (0=negative stain, 1=positive stain at <10%, 2=positive stain at 10–25%, 3=positive stain at >25%).
Reverse transcription quantitative polymerase chain reaction (qRT-PCR)
Total RNA was extracted from colon tissue samples using the RNeasy Mini kit (Qiagen, Valencia, California, USA) according to the manufacturer’s instructions. cDNA was synthesized from 1 μg RNA using AccuPower RT PreMix (Bioneer, Daejeon, Korea). qRT-PCR analysis of cDNA was performed using the ABI QuantaStudio 5 System (Applied Biosystems Japan, Tokyo, Japan) and the TaqMan Gene Expression Assays which included tumor necrosis factor (TNF)-α: Mm004 43258_m1, interleukin (IL)-1β: Mm00434228_m1, interferon (IFN)-γ: Mm00801778_m1, glyceraldehyde-3-phosphate dehydrogenase: Mm99999915_g1 (Applied Biosystems Japan). PCR conditions was 50°C for 2 min, 95°C for 10 min, followed by 40 cycles of 95°C for 15 sec and 60°C for 1 min.
Statistical analysis
Data were statistically compared using GraphPad Prism 5 (Graph Pad software, La Jolla, California, USA) through the Student’s t-tests. Differences with P-values <0.05 were considered significant. The results are expressed as the mean± standard error of mean.
RESULTS
Shape of EVs isolated from G. lamblia
EEVs, range from 30 to 1,000 nm, were known to modulate host-pathogen communication. EVs purified from
Giardia were observed using transmission electron microscopy (
Supplementary Fig. S1A). Representative TEM images showed that EV morphology was homogeneous with spherical shaped vesicles. Size of GlEVs was found to be between 100 and 200 nm in diameter (
Supplementary Fig. S1B).
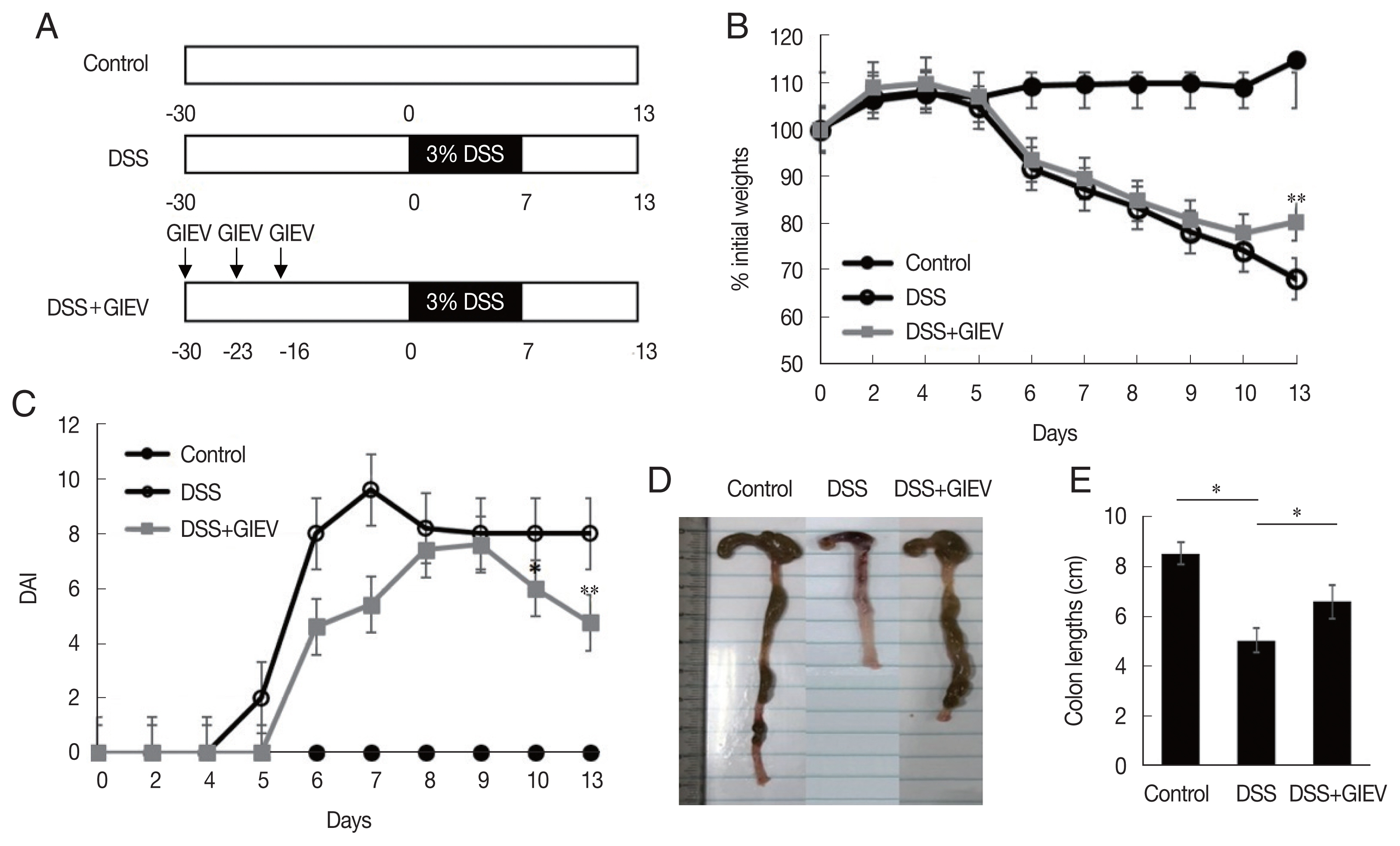
To determine whether GlEVs could prevent colitis, 10 μg of GlEVs was intraperitoneally injected into a mouse on days −30, −23, and −16 prior to induction of colitis by DSS (
Fig. 1A). While untreated mice maintained body weights during the experiment without significant changes, mice receiving 3% DSS markedly lost weights after 5 days. GlEV-treated mice also exhibited weight loss between days 5 and 10. However, they regained their weights between days 10 and 13 post-DSS induction (
Fig. 1B). Moreover, the DAI of GlEV-treated mice was lower than that in mice with DSS-induced colitis on day 5 after DSS treatment. Photographs of colon length from the different groups indicated that GlEV treatment prevented the colon shortening that is a typical symptom of colitis (
Fig. 1C, D). These results showed that DSS administration might induce inflammatory responses in mice, but these responses appeared to be preventable by the GlEV treatment.
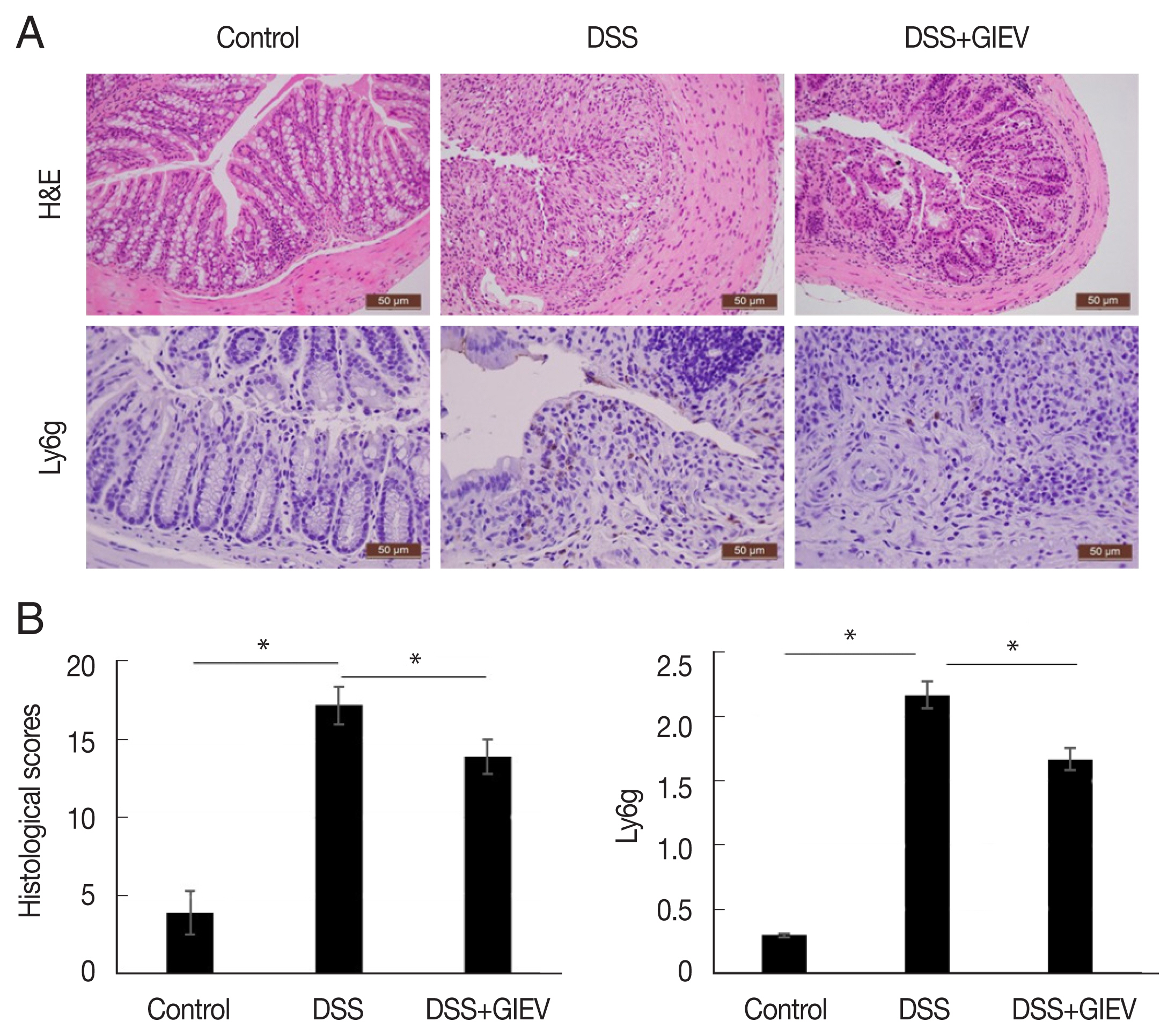
During intestinal inflammatory response, neutrophils migrate rapidly towards the inflammation site and gastric mucosal architecture with crypt abscesses and ulceration [
24]. In this study, the typical histological changes such as epithelial ulceration, severe inflammation, and loss of crypts were observed in mice administered with DSS (
Fig. 2A). However, treatment with GlEV in DSS-induced colitis mice showed reduced inflammation and crypt distortion. The histopathological score of GlEV-treated group (13.7 points) was 1.3-fold less than the DSS group (18.3 points). Additionally, to quantitate the inflammatory cell infiltration, the anti-Ly6g level were determined in colon tissue. Anti-Ly6g level was markedly increased in the DSS group (2.3 point) compared with that in the control (0.3 point) and DSS plus GlEV groups (1.6 point) was 1.4-fold less than the DSS group. These results indicated that GlEVs effectively suppressed infiltration of neutrophil (
Fig. 2B). These collective results suggested that GlEVs could attenuate the development of DSS-induced colitis.
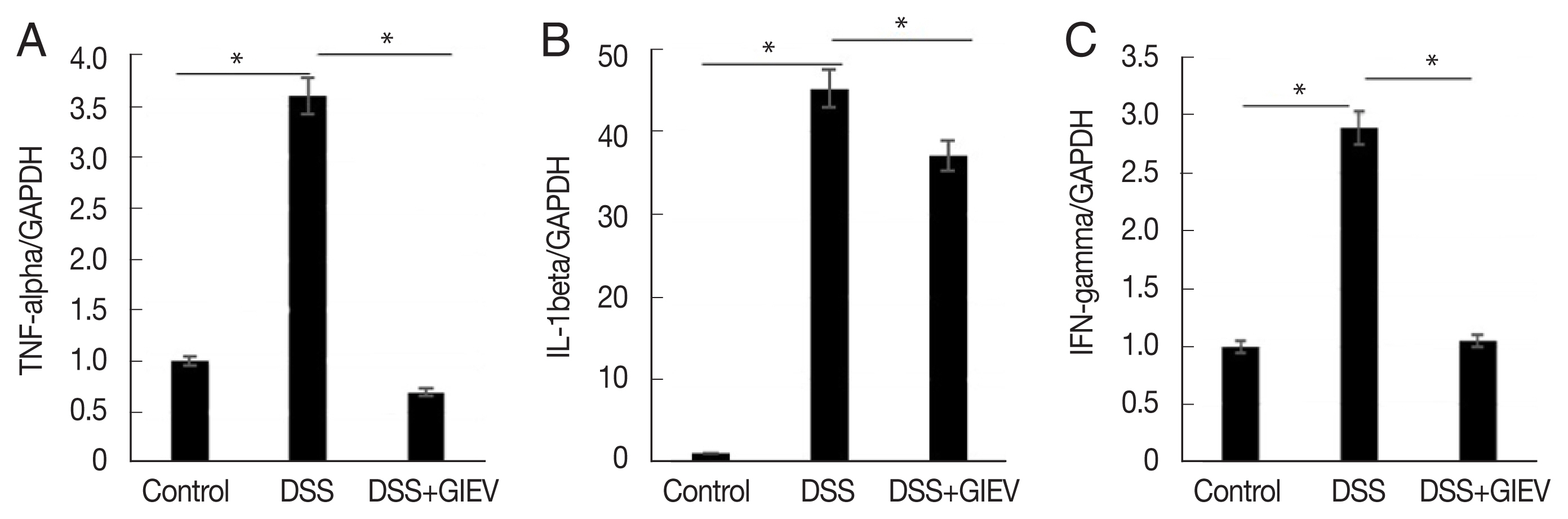
To assess whether GlEVs were involved in regulation of pro-inflammatory cytokine expression in the colons of mice with DSS-induced colitis, the levels of pro-inflammatory cytokines, including TNF-α, IL-1β, and IFN-γ, were determined using qRT-PCR. Pro-inflammatory cytokines were highly expressed in the DSS group compared with levels in the control group. However, pro-inflammatory cytokine expression levels were reduced in the DSS+GIEV group (
Fig. 3). These results indicated that GlEV potently regulated inflammatory cytokine production in DSS-induced colitis.
DISCUSSION
Giardiasis induces diarrhea in infected hosts including humans. Most studies conducted on the analysis of the role of
Giardia pathogenesis focus on the crosstalk between the host and
Giardia-derived molecules that can lead to the development of diseases [
17]. For example,
Giardia arginine deiminase and ornithine carbamoyl transferase were reported to be virulence factors, which play a role in the inhibition of cell proliferation [
21,
22]. In contrast, some studies revealed the ability of
Giardia to act as an immune modulator in inflammatory disease. The lysates of
Giardia trophozoites can attenuated lipopolysaccharide-induced inflammatory cytokine expression in human dendritic cells by upregulating expression levels of IL-10 [
19].
Giardia trophozoites also suppress the IL-1β-induced expression of chemokine (C–X–C motif) ligand 8 (CXCL8) in vitro and ex vivo in human tissues obtained from patient with Crohn’s disease [
18]. Giardia cathepsin B can degrade CXCL8 expressed by Caco-2 cells in response to pro-inflammatory stimuli [
23]. Previous research has identified 154 proteins of GlEVs, which included α-tubulin, heat shock proteins, and variant surface protein, giardin, and arginine deiminase. They have been reported to play a role in biogenesis of EVs and
Giardia pathogenesis [
31]. In this study, we found that GlEVs exerted a potent effect on the immune response in mice with DSS-induced colitis.
The DSS-induced colitis mouse model is widely used for IBD research because the clinical and histological signs of disease are similar to those of human IBD, including diarrhea, weight loss, and colon shortening. Although the pathogenesis of colitis caused by DSS is not clear, it is due to 1) reduction and death of mucosal epithelial cells via an increase in the osmotic rate of the colonic mucosa, 2) intestinal tract inflammation triggered by intestinal specific T cell induction of the immune inflammatory response, 3) increased expression of cytokines such as TNF-α and INF-γ, and 4) changes in microflora environment. Mice with DSS-induced colitis have been reported to exhibit higher disease activity and weight loss than normal mice [
24,
25]. The administration of DSS via drinking water to C57BL/6 mice for 7 days induced colitis, which invoked diarrhea, bloody feces, weight loss, and inflammatory histological changes in colon tissue. However, the GlEV-treated group regained weights and showed reduced clinical signs, as well as reduced inflammatory cell infiltration. Our results suggest that GlEV treatment for the simplicity and controllability of the DSS-induced colitis model.
The balance of inflammatory cells is essential for the maintenance of normal gut homeostasis [
26]. The role of pro-inflammatory cytokines, such as TNF-α, IL-1β, and IFN-γ in IBD, is correlated with the initiation and progression of IBD pathogenesis [
27,
28]. TNF-α promotes inflammatory processes through increased expression of IL-1β and IFN-γ, as well as related cytotoxic effects and apoptosis in inflammatory lesions. TNF-α levels in the serum correlate with the disease activity of IBD. Similarly, IL-1β is a key player in the development of intestinal inflammation and can lead to T cell responses such as Th17 cell differentiation and IFN-γ expression [
29,
30]. It exerts major inflammatory effects on intestinal epithelial cells, disrupting goblet cell maturation and functions. We also investigated the effect of GlEV treatment on cytokine expression in the colons of mice with DSS-induced colitis. When mice were treated with GlEV, decreased expression of pro-inflammatory cytokines, such as TNF-α, IL-1β, and IFN-γ, compared with that in the DSS group were observed.
In conclusion, our study demonstrates that GlEVs ameliorate clinical and histological features of colitis and attenuates DSS-induced colitis by decreasing the expression levels of pro-inflammatory cytokines. This study shows that EVs from G. lamblia can exert preventive effects on DSS-induced colitis, raising a possibility as a therapeutic agent against IBD.
Notes
-
The authors declare no competing interest. The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Supplementary Information
ACKNOWLEDGMENT
This work was supported by a grant from the Korea Centers for Disease Control and Prevention (2017-NC54001-02).
Fig. 1Treatment with mice with Giardia lamblia extracellular vesicles (GlEVs) ameliorates clinical signs and colon shortening in dextran sulfate sodium (DSS)-induced acute colitis. (A) Schematic time schedule of immunization with GlEVs and DSS induction of colitis in C57BL/6 mice. (B) The body weights were determined daily for each mouse and expressed as the percentage of initial weights. (C) The disease activity index (DAI) was evaluated daily using the parameters of weight loss, stool consistency, and fecal blood. (D, E) Colon lengths. Each group consisted of 5 mice (n=3). Asterisks indicate that statistical difference between 2 groups. *P<0.05; **P<0.01.
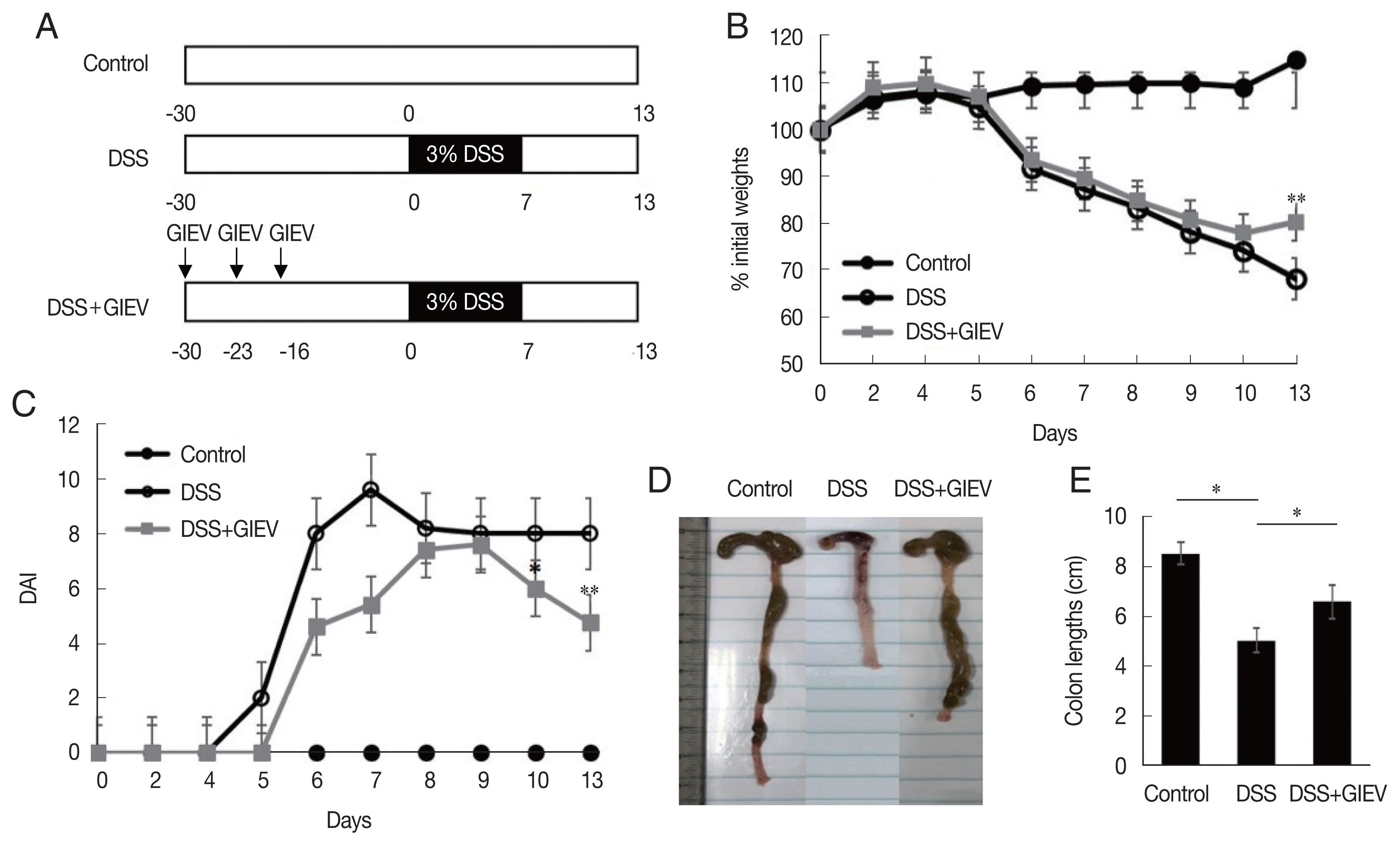
Fig. 2Histological and immunohistochemical staining of DSS-induced colitis. (A) Colon tissue samples were examined using hematoxylin–eosin staining and immunohistochemical staining with an anti-Ly6g antibody. (B) Representative histopathological and immunostaining score of colon tissue. Each group consisted of 5 mice (n=3). *P<0.05.
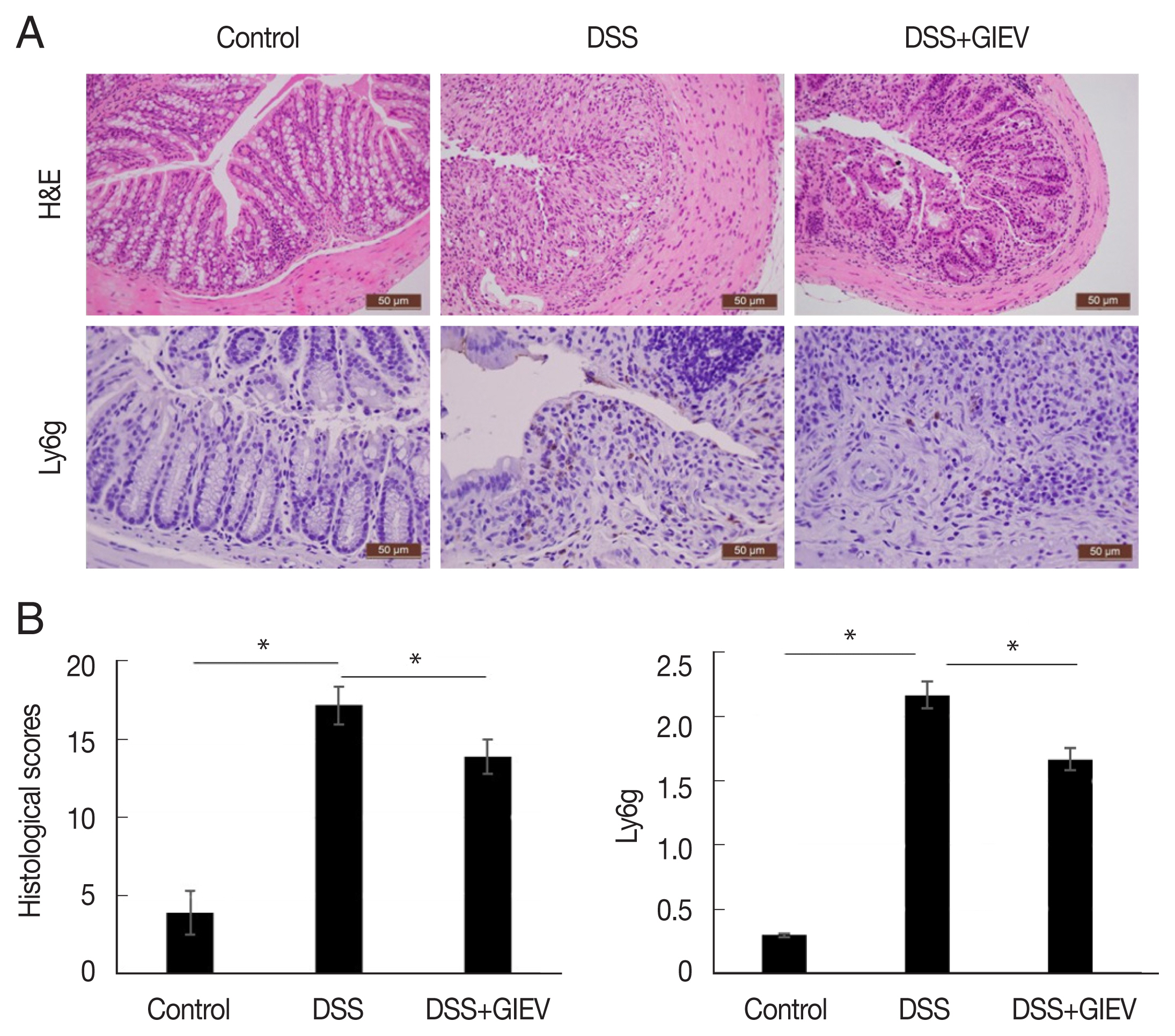
Fig. 3
Giardia lamblia extracellular vesicles (GlEVs) suppress the expression of pro-inflammatory cytokines in colons of mice with DSS-induced colitis. (A–C) The expression levels of pro-inflammatory cytokines, such as tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and interferon (IFN)-γ, analyzed using reverse transcription quantitative polymerase chain reaction were lower in the GlEV-treated group (DSS +GlEV) than in the DSS-induced group (DSS). Each group consisted of 5 mice (n=3). *P<0.05.

References
- 1. Kornbluth A, Sachar DB. Ulcerative colitis practice guidelines in adults: American college of gastroenterology, practice parameters committee. Am J Gastroenterol 2010;105:501-523. https://doi.org/10.1038/ajg.2009.727
- 2. Ng WK, Wong SH, Ng SC. Changing epidemiological trends of inflammatory bowel disease in Asia. Intest Res 2016;14:111-119. https://doi.org/10.5217/ir.2016.14.2.111
- 3. Zhang YZ, Li YY. Inflammatory bowel disease: pathogenesis. World J Gastroenterol 2014;20:91-99. https://doi.org/10.3748/wjg.v20.i1.91
- 4. Pithadia AB, Jain S. Treatment of inflammatory bowel disease (IBD). Pharmacol Reports 2011;63:629-642. https://doi.org/10.1016/s1734-1140(11)70575-8
- 5. Hemperly A, Sandborn WJ, Vande Casteele N. Clinical pharmacology in adult and pediatric inflammatory bowel disease. Inflamm Bowel Dis 2018;24:2527-2542. https://doi.org/10.1093/ibd/izy189
- 6. Rawla P, Sunkara T, Raj JP. Role of biologics and biosimilars in inflammatory bowel disease: current trends and future perspectives. J Inflamm Res 2018;11:215-226. https://doi.org/10.2147/JIR.S165330
- 7. Zuo T, Ng SC. The gut microbiota in the pathogenesis and therapeutics of inflammatory bowel disease. Front Microbiol 2018;9:2247. https://doi.org/10.3389/fmicb.2018.02247
- 8. Versini M, Jeandel PY, Bashi T, Bizzaro G, Blank M, Shoenfeld Y. Unraveling the hygiene hypothesis of helminthes and autoimmunity: origins, pathophysiology, and clinical applications. BMC Med 2015;13:81. https://doi.org/10.1186/s12916-015-0306-7
- 9. Smallwood TB, Giacomin PR, Loukas A, Mulvenna JP, Clark RJ, Miles JJ. Helminth immunomodulation in autoimmune disease. Front Immunol 2017;8:453. https://doi.org/10.3389/fimmu.2017.00453
- 10. Summers RW, Elliot DE, Urban JF, Thompson R, Weinstock JV. Trichuris suis therapy in Crohn’s disease. Gut 2005;54:87-90. https://doi.org/10.1136/gut.2004.041749
- 11. Liu Y, Ye Q, Liu YL, Kang J, Chen Y, Dong WG. Schistosoma japonicum attenuates dextran sodium sulfateinduced colitis in mice via reduction of endoplasmic reticulum stress. World J Gastroenterol 2017;23:5700-5712. https://doi.org/10.3748/wjg.v23.i31.5700
- 12. Hang L, Setiawan T, Blum AM, Urban J, Stoyanoff K, Arihiro S, Reinecker H, Weinstock JV. Heligmosomoides polygyrus infection can inhibit colitis through direct interaction with innate immunity. J Immunol 2010;185:3184-3189. https://doi.org/10.4049/jimmunol.1000941
- 13. Roig J, Saiz ML, Galiano A, Trelis M, Cantalapiedra F, Monteagudo C, Giner E, Giner RM, Recio MC, Bernal D, Sanchez-Madrid F, Marcilla A. Extracellular vesicles from the helminth Fasciola hepatica prevent DSS-induced acute ulcerative colitis in a T-lymphocyte independent mode. Front Microbiol 2018;9:1036. https://doi.org/10.3389/fmicb.2018.01036
- 14. Riveau G, Deplanque D, Remoué F, Schacht A, Vodougnon H, Capron M, Thiry M, Martial J, Libersa C, Capron A. Safety and immunogenicity of rSh28GST antigen in humans: phase 1 randomized clinical study of a vaccine candidate against urinary schistosomiasis. PLoS Negl Trop Dis 2012;6:e1704. https://doi.org/10.1371/journal.pntd.0001704
- 15. Driss V, El Nady M, Delbeke M, Rousseaux C, Dubuquoy C, Sarazin A, Gatault S, Dendooven A, Riveau G, Colombel JF, Desreumaux P, Dubuquoy L, Capron M. The schistosome glutathione S-transferase P28GST, a unique helminth protein, prevents intestinal inflammation in experimental colitis through a Th2-type response with mucosal eosinophils. Mucosal Immunol 2016;9:322-335. https://doi.org/10.1038/mi.2015.62
- 16. Ma'ayeh SY, Liu J, Peirasmaki D, Hörnaeus K, Bergström Lind S, Grabherr M, Bergquist J, Svärd SG. Characterization of the Giardia intestinalis secretome during interaction with human intestinal epithelial cells: the impact on host cells. PLoS Negl Trop Dis 2017;11:e0006120. https://doi.org/10.1371/journal.pntd.0006120
- 17. Emery SJ, Mirzaei M, Vuong D, Pascovici D, Chick JM, Lacey E, Haynes PA. Induction of virulence factors in giardia duodenalis independent of host attachment. Sci Rep 2016;6:1-16. https://doi.org/10.1038/srep20765
- 18. Cotton JA, Motta JP, Schenck LP, Hirota SA, Beck PL, Buret AG. Giardia duodenalis infection reduces granulocyte infiltration in an in vivo model of bacterial toxin-induced colitis and attenuates inflammation in human intestinal tissue. PLoS One 2014;9:1-15. https://doi.org/10.1371/journal.pone.0109087
- 19. Summan A, Nejsum P, Williams AR. Modulation of human dendritic cell activity by Giardia and helminth antigens. Parasite Immunol 2018;40:e12525. https://doi.org/10.1111/pim.12525
- 20. Kim JJ, Shajib MS, Manocha MM, Khan WI. Investigating intestinal inflammation in DSS-induced model of IBD. J Vis Exp 2012;1:1-6. https://doi.org/10.3791/3678
- 21. Stadelmann B, Merino MC, Persson L, Svard SG. Arginine consumption by the intestinal parasite Giardia intestinalis reduces proliferation of intestinal epithelial cells. PLoS One 2012;7:e45325. https://doi.org/10.1371/journal.pone.0045325
- 22. Banik S, Viveros PR, Seeber F, Klotz C, Ignatius R, Aebischer T. Giardia duodenalis arginine deiminase modulates the phenotype and cytokine secretion of human dendritic cells by depletion of arginine and formation of ammonia. Infect Immun 2013;81:2309-2317. https://doi.org/10.1128/IAI.00004-13
- 23. Cotton JA, Bhargava A, Ferraz JG, Yates RM, Beck PL, Buret AG. Giardia duodenalis cathepsin B proteases degrade intestinal epithelial interleukin-8 and attenuate interleukin-8-induced neutrophil chemotaxis. Infect Immun 2014;82:2772-2787. https://doi.org/10.1128/IAI.01771-14
- 24. Chassaing B, Aitken JD, Malleshappa M, Vijay-Kumar M. Dextran sulfate sodium (DSS)-induced colitis in mice. Curr Protoc Immunol 2014;104:1-15. https://doi.org/10.1002/0471142735.im1525s104
- 25. Alex P, Zachos NC, Nguyen T, Gonzales L, Chen TE, Conklin L, Centola M, Li X. Distinct cytokine patterns identified from multiplex profiles of murine DSS and TNBS-induced colitis. Inflamm Bowel Dis 2009;15:341-352. https://doi.org/10.1002/ibd.20753
- 26. Friedrich M, Pohin M, Powrie F. Review cytokine networks in the pathophysiology of inflammatory bowel disease. Immunity 2019;50:992-1006. https://doi.org/10.1016/j.immuni.2019..017
- 27. Lee SH, Kwon JE, Cho ML. Immunological pathogenesis of inflammatory bowel disease. Intest Res 2018;16:26-42. https://doi.org/10.5217/ir.2018.16.1.26
- 28. Sanchez-Muñoz F, Dominguez-Lopez A, Yamamoto-Furusho JK. Role of cytokines in inflammatory bowel disease. World J Gastroenterol 2008;14:4280. https://doi.org/10.3748/wjg.14.4280
- 29. Noti M, Corazza N, Mueller C, Berger B, Brunner T. TNF suppresses acute intestinal inflammation by inducing local glucocorticoid synthesis. J Exp Med 2010;207:1057-1066. https://doi.org/10.1084/jem.20090849
- 30. Levin AD, Wildenberg ME, van den Brink GR. Mechanism of action of anti-TNF therapy in inflammatory bowel disease. J Crohns Colitis 2016;10:989-997. https://doi.org/10.1093/ecco-jcc/jjw053
- 31. Zhao P, Cao L, Wang X, Dong J, Zhang N, Li X, Li J, Zhang X, Gong P. Extracellular vesicles secreted by Giardia duodenalis regulate host cell innate immunity via TLR2 and NLRP3 inflammasome signaling pathways. PLoS Negl Trop Dis 2021;15:e0009304. https://doi.org/10.1371/journal.pntd.0009304