Abstract
Reptiles were known to serve as paratenic hosts for Centrorhynchus (Acanthocephala: Centrorhynchidae) in Korea, but the infection course in experimental animals was not elucidated yet. In this study, the tiger keelback snakes (Rhabdophis tigrinus) were collected and digested with artificial pepsin solution, and the larvae of Centrorhynchus were recovered from them. Then, the collected larvae were orally infected to rats for developmental observations. In rats, all the larvae were observed outside the intestine on day 3 post-infection (PI), including the mesentery and abdominal muscles. As for the development in rats, the ovary of Centrorhynchus sp. was observed at day 15 PI, and the cement glands were 3 in number. Based on the morphological characteristics, including the arrangement of proboscis hooks, these larvae proved to be a species of Centrorhynchus, and more studies were needed for species identification.
-
Key words: Centrorhynchus, acanthocephala, migration, Rhabdophis tigrinus, development
INTRODUCTION
Acanthocephalan worms are endoparasites of animals, forming a unique phylum by virtue of their structure and extreme parasitic habits [
1]. Their life cycles usually have insects or isopods for intermediate hosts [
2], and the definitive hosts vary according to the species. Although acanthocephalans can develop infectivity in vertebrates via arthropod intermediate hosts, another vertebrate host occurs between the arthropod intermediate host and the vertebrate definitive host in some species [
2]. Amphibians and reptiles serve as paratenic hosts for some acanthocephalan species that mature in flesh-eating birds, and species of
Centrorhynchus are known to be present in frogs, lizards, and snakes [
2].
The acanthocephalans are distributed worldwide [
3], and several reports on the presence of acanthocephalans had existed in Korea. The adult worms of
Echinorhynchus gadi were discovered in changran-pickles and myungran-pickles [
4], and larvae of
Centrorhychus sp. were from the mesentery of tiger keelback snakes,
Rhabdophis tigrinus [
5]. Although there have been no reports on human infection with
Centrorhynchus sp. in Korea, accidental infection with
Centrorhynchus may occur considering that some of Koreans eat the snakes raw. Hence, it is needed to observe the outcome of acanthocephalan growth and development in experimental animals. This study was conducted to know the infection density of
Centrorhynchus sp. in tiger keelback snakes and to describe their biological characteristics by experimental infection to rats.
MATERIALS AND METHODS
Examination of snakes for presence of Centrorhynchus larvae
Between March and May 2009, 20 tiger keelback snakes (R. tigrinus) were collected from Ganghwa-gun, Incheon-si, Korea. After peeling off the snake skin, the viscera were separated from the muscle. Then, the viscera were cut into small segments, and were digested with artificial digestive juice for 1 hr at 36℃. They were washed several times with phosphate buffered saline (PBS) and investigated for the presence of Centrorhynchus under a stereomicroscope. The number of recovered larvae was counted per snake, and some of them were fixed in formalin for microscopic examinations.
Experimental infection and recovery of Centrorhynchus sp.
The collected larvae (days 0 PI) were used for experimental infection in rats, and some were fixed in 10% formalin for microscopic examinations. Twenty Sprague-Dawley rats (4 weeks old) were experimentally fed 70 larvae each, and sacrificed by cervical dislocation on days 1, 3, 5, 10, 15, 20, and 70 PI. The number of sacrificed rats was 4 except for days 1, 3, and 70 PI, in which 1-2 rats were sacrificed. The rats were thoroughly investigated for Centrorhynchus larvae, especially in the abdominal cavity. The intestines were also examined, and the viscera containing the worms were fixed in 10% neutral formalin and stained in hematoxylin-eosin for histological sections. The recovered worms were fixed with 10% neutral formalin under cover slip pressure, and were examined after Semichon's acetocarmine staining.
RESULTS
Recovery of Centrorhynchus larvae from rats
All the snakes (
R. tigrinus) examined were infected with
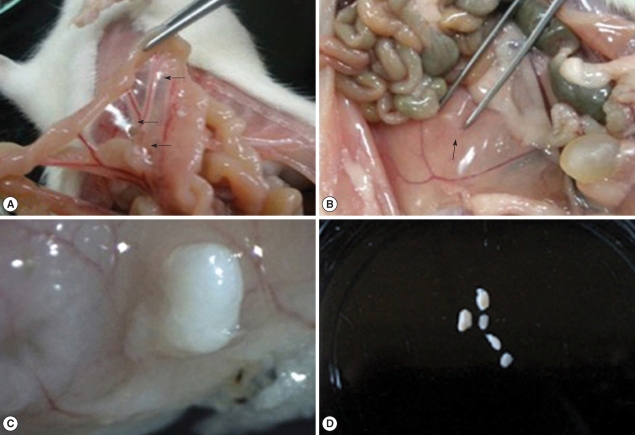
Centrorhynchus larvae (100%), and the number of larvae was in the range from 11 to 266 (120.6 ± 70.0). In experimentally infected rats, the recovery rate of larvae was maintained in the range of 25-37% except for 14.3% of day 3 PI. The larvae were found in extraintestinal sites on day 1 PI, and all the larvae were discovered outside the intestine on day 3 PI (
Table 1). The mesentery was the most preferred site (
Fig. 1A), and some of the worms were observed in the muscles of the rats (
Fig. 1B). On day 3 PI, the penetrating larva was observed in the intestinal wall (
Fig. 1C). All the extraintestinal worms were surrounded by a fibrotic capsule (
Fig. 1D).
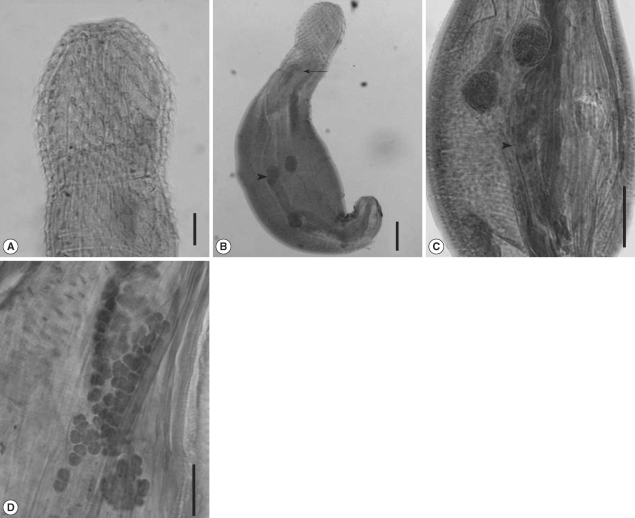
The trunk was robust, aspinose, and pyriform. The maximum width was located slightly posterior to the midline. The recovered larvae from the snakes were 1,398 × 733.9 µm in size, and they became slender as infection proceeds, 2,632.0 × 787.5 µm on day 20 PI. The female worm was larger than the male worm in average size. Two tubular lemnisci were on both sides of the proboscis, forming a sac-like structure. The proboscis was entirely covered with hooks. The number of hooks was nearly unchanged according to the developmental days, 29-33 longitudinal rows of 12-13 hooks (
Fig. 2A). The proboscis receptacle was clearly distinguished on day 3 PI. It consisted of a double-layered wall, a ventral one more attenuated than the dorsal one. Numerous muscle fibers were seen in these walls, and the proboscis retractor muscle was located inside it. The proboscis receptacle tapered into the posterior end, forming a blind sac. Beneath the proboscis, 2 lemnisci began on both sides of the proboscis, ending anterior to the proboscis receptacle.
In male worms, two testes were round and equal in size, arranged tandem. They were found in the posterior 4/5 on day 1 PI, but moved to the equator on day 3 PI (
Fig. 2B), finally located at the lower border of lemnisci. The Safftigen's pouch was observed at the posterior part, and the copulatory bursa was extruded on day 3 PI. The cement glands were tubular, posterior to the testis. On day 20 PI, numerous cells were observed inside the cement gland, composed of 3 tubular structures in male worms (
Fig. 2C).
The female worm had a uterine bell near the posterior end of the trunk, connected with an egg sorting apparatus, uterus, vagina, and vulva, which opens terminally. The vagina was surrounded by a muscle sphincter, which was divided into 2 portions (anterior and posterior). Ovary developed as numerous cell aggregates, floating in the body cavity (
Fig. 2D). From these findings, it was concluded that this parasite belonged to the phylum Acanthocephala, especially the genus
Centrorhynchus.
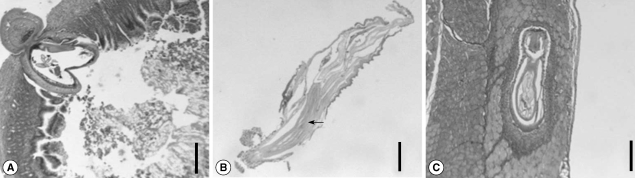
On the day 1 PI, it was observed that a larva was penetrating the intestinal wall (
Fig. 3A). The anterior part of the worm was already outside the wall. Small intestinal mucosa revealed acute inflammatory infiltrates around the worm (
Fig. 3A). The widening of lamina propria and villi blunting were also observed. On day 5 PI, the larvae were also penetrating the wall, but some were entering the muscles of rats. On day 15 PI, the section of male worms revealed 3 tubular structures of cement glands (
Fig. 3B). On day 20 PI, some larvae were observed in the internal oblique muscle of rats (
Fig. 3C). It was observed that the worm was rimmed by epithelioid histiocytes with infiltration of lymphocytes and eosinophils, forming vague granulomas. The remaining skeletal muscle showed no specific changes (
Fig. 3C).
DISCUSSION
From this study, the tiger keelback snakes (
R. tigrinus) were proved to be a paratenic host for
Centrorhynchus sp. in Korea. The infection density of the snakes in Ganghwa-gun, Inchoensi, was 120.6 larvae per snake, higher than 34.6 of Yeongdong-gun, Chungcheongbuk-do [
5]. For an epizootiological survey, snakes of other regions should be surveyed. In addition, other kinds of animals should also be examined because the larvae of
Centrorhynchus are known to infect invertebrates, amphibians, reptiles, or mammals [
6].
The larvae were belonged to the genus
Centrorhynchus in terms of various morphological characteristics, especially the arrangement of proboscis hooks. The proboscis hooks of
Centrorhynchus sp. were 16-48 longitudinal rows of 8-40 each [
6]. Especially, it was 28-29 longitudinal rows of 15-16 hooks in
Centrorhynchus aluconis [
7], 30-32 rows of 12 in
Centrorhynchus cinctum [
8], similar to 29-33 × 12-13 in the present worms. In addition, the testes were in the anterior portion of the trunk, and the number of cement glands was 3, fitting the criteria of 3-6 [
6]. However, the detailed species identification was not possible due to the absence of adult worms. Since the adults of this genus are known to be parasitic in birds [
6], an experimental infection in birds is needed for species identification.
It was also shown that
Centrorhynchus larvae underwent extraintestinal migration immediately after oral infection in rats. The same phenomenon might occurr in human infections, and it was suspected that these parasites could evoke a gastrointestinal disturbance as seen in
Bolbosoma infection [
9]. Since it was proved that the formation of granulomas by the worm was possible in the abdominal muscle,
Centrorhynchus sp. infection should be considered in a patient complaining of abdominal pain of unknown origin.
There have been few reports on acanthocephalans in Korea. The research on the parasites of this phylum is needed not only to expand the parasite fauna in Korea, but to contribute to the health of the people. In this study, the detailed species identification was not possible due to the absence of full development and intrauterine eggs of the worm.
Centrorhynchus is the largest acanthocephalan genus having almost 90 species, and these are parasites mainly of birds of the orders Falconiformes and Strigiformes [
7]. Hence, an experimental infection into birds should be the first target to be examined.
ACKNOWLEDGEMENTS
This work was funded by Medical Science Research Center of Dankook University Medical Center in 2008.
References
- 1. Sahar MM, Madani TA, Al Mohsen IZ, Almodovar EL. A child with an acanthocephalan infection. Ann Saudi Med 2006;26:321-324.
- 2. Atkinson CT, Thomas NJ, Hunter DB. Parasitic Diseases of Wild Birds. 2008, Ames, Iowa, USA. Wiley-Blackwell; p 279.
- 3. Roberts L, Janovy JR. Foundation of Parasitology. 2005, 7th ed. Boston, USA. McGraw-Hill; pp 493-509.
- 4. Kim JT, Park JY, Seo HS, Oh HG, Noh JW, Kim SW, Youn HJ. Identification of Acanthocephala discovered in changran-pickles and myungran-pickles. J Vet Sci 2001;2:111-114.
- 5. Shin DW, Shim UT. Studies on the cystacanth of acanthocephala from the mesentery of Natrix tigrina. Chungnam Med J 1984;11:23-33.
- 6. Yamaguti S. Systema helminthum. Acanthocephala. 1963, Vol. 5:New York, USA. John Wiley & Sons Inc; pp 112-129.
- 7. Dimitrova ZM, Gibson DI. Some species of Centrorhynchus Lűhe, 1911 (Acanthocephala: Centrorhynchidae) from the collection of the Natural History Museum, London. Syst Parasitol 2005;62:117-134.
- 8. Meyer A. In Bronns Hg ed, Acanthocephala. Klassen und Ordnungen des Tierreichs. Band 4. Abteilung 2. Buch 2. 1933, Leipzig. Akademische Verlagsgesellscahft M.B.H; pp 1-582.
- 9. Beaver PC, Otsuji T, Otsuji A, Yoshimura H, Uchikawa R, Sato A. Acanthocephalan, probably Bolbosoma, from the peritoneal cavity of man in Japan. Am J Trop Med Hyg 1983;32:1016-1018.
Fig. 1Acanthocephalan larvae are observed in an infected rat. (A) Three larvae are observed in the mesentery of a rat on day 5 PI (arrows). (B) An acanthocephalan larva is embedded in the internal oblique muscle of a rat on day 20 PI (arrow). (C) An acanthocephalan larva is penetrating the intestinal wall of a rat on day 3 PI. Stereomicroscopic view. (D) Five acanthocephalan larvae from a rat on day 70 PI, surrounded by the cyst.
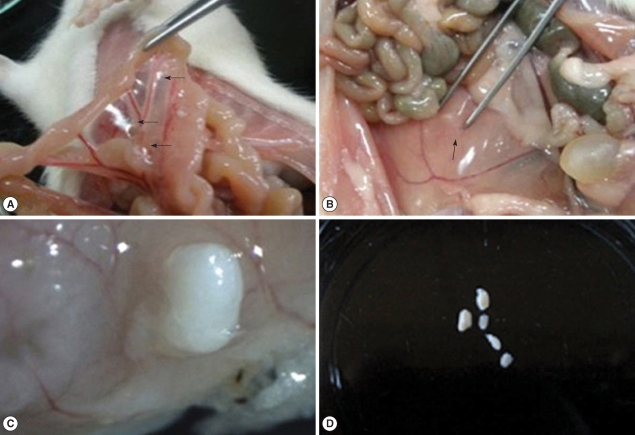
Fig. 2(A) The anterior part of a 10 day-old male. The number of hooks is 30 longitudinal rows of 13 hooks. (B) A 3-day old male. The proboscis receptacle is seen (arrowhead), and 2 testes are located near the midline of the worm (arrow). Bar = 10 µm. (C) A 20-day old male, showing 2 testes (arrow) and cement glands (arrowhead). Numerous cells are observed inside the cement gland. (D) A 20-day female larva showing numerous floating ovaries. Bar = 100 µm.
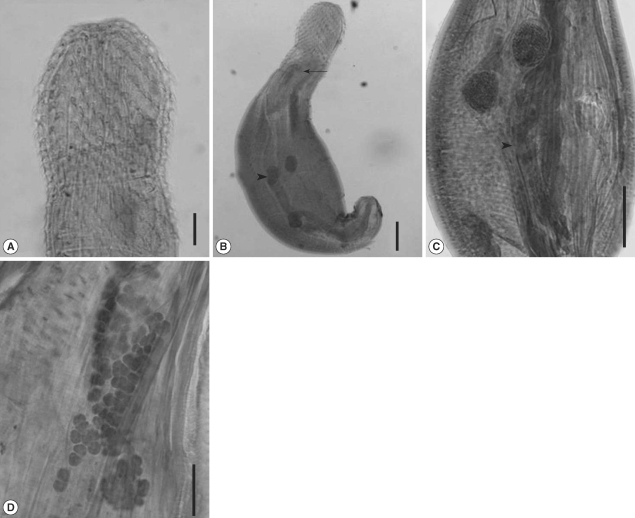
Fig. 3Section of the intestine and abdominal muscle of a rat. (A) A larva is penetrating the intestinal wall on day 1 PI. The anterior part of the worm was already outside the wall. Acute inflammatory infiltrates are observed around the worm. Bar = 400 µm. (B) Section of a 15-day old male, showing 3 tubular structures of cement glands. The testes are also seen anteroventrally. Bar = 200 µm. (C) A larva is observed in the internal oblique muscle of a rat on day 20 PI. It was observed that the worm was rimmed by epithelioid histiocytes with infiltration of lymphocytes and eosinophils, forming indistinct granulomas. Bar = 250 µm.
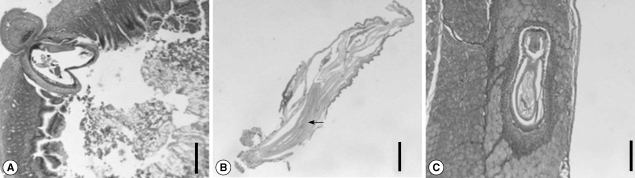
Table 1.Number of Centrorhynchus larvae recovered from rats with reference to the distribution
Table 1.
|
Days PI (post-infection) |
Intestinal lumen |
Intestinal wall |
Mesentery |
Muscle |
Total no. (%) of worms |
|
1 |
27.0 |
5.0 |
5.0 |
0.0 |
37.0 (52.8) |
|
3 |
1.0 |
0.5 |
8.5 |
0.0 |
10.0 (14.3) |
|
5 |
0.0 |
4.5 |
24.8 |
1.3 |
30.5 (43.6) |
|
10 |
0.0 |
2.0 |
26.8 |
0.3 |
26.5 (41.4) |
|
15 |
0.0 |
1.3 |
27.0 |
0.5 |
31.2 (44.6) |
|
20 |
0.0 |
0.8 |
32.3 |
0.8 |
34.5 (49.3) |
|
70 |
0.0 |
0.0 |
26.0 |
1.0 |
27.0 (38.6) |