Abstract
It is uncertain when the head collar and collar spines of Isthmiophora hortensis (Digenea: Echinostomatidae), a zoonotic echinostome species in Far Eastern Asia, develop during its larval stages. In this study, the appearance of the head collar and collar spines was studied using light and scanning electron microscopy in cercariae and metacercariae experimentally obtained from freshwater snails (Lymnaea pervia) and tadpoles (Rana nigromaculata), respectively. The cercariae were shed from the snail on day 30 after exposure to laboratory-hatched miracidia. Metacercariae were obtained from the experimental tadpoles at 3, 6, 12, 15, 20, 24, 26, and 30 h after exposure to the cercariae. The head collar was already visible in the cercarial stage, although its degree of development was weak. However, collar spines did not appear in the cercarial stage and even in the early metacercarial stage less than 24 h postinfection in tadpoles. Collar spines became visible in the metacercariae when they grew older than 24 h. It was concluded that the head collar of I. hortensis developed early in the cercarial stage, but the development of collar spines did not occur until the worms became 24-h-old metacercariae in our experimental setting. Counting the number of collar spines was concluded as an unfeasible diagnostic method for I. hortensis cercariae when they are shed from the snail host.
-
Key words: Isthmiophora hortensis, head collar, collar spine, cercaria, metacercaria
Isthmiophora hortensis (Asada, 1926) Kostadinova and Gibson, 2002 (syn.
Echinostoma hortense Asada, 1926;
Echinostoma campi Ono, 1930) was originally reported from experimental rats and dogs fed metacercariae in tadpoles of
Rana sp. and
Bufo sp. in Japan [
1,
2]. Natural infections of mice, rats, and humans have been reported in Korea, Japan, and China [
2]. In Korea, human infections are common in endemic areas, such as Cheongsong-gun, Gyeongsangbuk-do (Province) and Geochang-gun, Gyeongsangnam-do, with egg-positive rates of 22.4% and 9.5%, respectively, in fecal examinations [
3,
4]. In addition, cases with clinical complaints of severe abdominal discomfort were reported from various localities in Korea and Japan; the patients were diagnosed by detecting adult flukes attached to the intestinal mucosa by gastroduodenal endoscopy or colonoscopy [
2]. The first intermediate host is freshwater snails of
Lymnaea and
Radix spp., namely,
L. pervia,
L. ollula,
L. japonica, and
R. auricularia coreana [
2,
5]. The second intermediate host is the amphibians, including tadpoles of
Rana or
Bufo spp. and larvae of
Hynobius spp., and freshwater fish, such as
Odontobutis spp., including
O. obscura interrupta,
Moroco oxycephalus (=
Rhynchocypris oxicephalus),
Squalidus japonicus coreanus,
Misgurnus anguillicaudatus, and
Carassius auratus [
2,
6].
Adults of
I. hortensis are morphologically characterized by the presence of a head collar (=head crown) equipped with 27–28 dorsally uninterrupted collar spines in 2 alternating rows (including 4 corner spines on each side) around the oral sucker [
2,
7]. Thus, these morphological features can be used as a diagnostic criterion for differentiating
I. hortensis from other echinostome species (adults and larvae) occurring in Korea;
Echinostoma cinetorchis and
Echinochasmus japonicus have 37 (5 corner spines on each side) and 24 dorsally interrupted collar spines (without corner spines), respectively [
2].
However, in
I. hortensis, it is uncertain at which point the development of the head collar and collar spines begins during their larval stages. Ono [
8,
9] and Yamaguti [
10] described the cercarial morphologies of
I. hortensis (under the names
Echinostoma campi [
8,
9] and
Echinostoma hortense [
10]), stating that the head collar and collar spines were well-developed in the cercarial stage with supportive figure drawings. However, Okamoto [
11] reported that the collar spines in
I. hortensis (under the name
Echinostoma hortense) did not develop in the cercarial stage. Moreover, during the life cycle studies of
I. hortensis by our group (under the name
Echinostoma hortense) [
12], we could not observe the development of collar spines in the cercarial stage. This point is important in the morphological diagnosis of echinostome cercariae shed from lymnaeid snails, and the debates on collar spine development need to be clarified. In the present study, we aimed to observe the development of the head collar and collar spines of
I. hortensis during the larval stages (cercariae and metacercariae) obtained in the laboratory through light (LM) and scanning electron microscopy (SEM) observations. This is a further in-depth study associated with the laboratory maintenance of the life cycle by Lee et al. [
12] and a summarized report of a M.S. thesis by Jung [
13].
The cercariae shed from laboratory-passaged (>1 year)
Lymnaea pervia snails were collected under a stereomicroscope at day 30 post-exposure to miracidia hatched from the eggs laid by 3-week-old adult flukes grown in experimentally infected Sprague-Dawley rats [
13]. The animal experiments were performed following the ethical guidelines of the Institutional Animal Care and User Committee at Seoul National University College of Medicine (At the time of this experiment in 1990–1991, there was no institutional review board on animal experiments in our university). Some of the cercariae were placed in a dish containing laboratory-reared tadpoles of
Rana nigromaculata to obtain the metacercariae. The remaining cercariae were fixed in 10% neutral buffered formalin (hot) for LM and 2.5% glutaraldehyde for SEM studies. The formalin-fixed cercariae (unstained) were placed on a glass slide under a cover slip and directly observed using an LM (Olympus, Tokyo, Japan).
The infected tadpoles were killed at 3, 6, 12, 15, 20, 24, 26, and 30 h after exposure to the cercariae, and the metacercariae from different infection ages were harvested from the gills and posterior trunk muscles by the slide compression method [
13]. Some metacercariae were excysted by incubating them in a 2% trypsin solution [
13]. The encysted (
n=10) and excysted (
n=10) metacercariae from different infection ages (a total of 160 metacercariae) were placed on a glass slide with a cover slip and observed using an LM. Some excysted metacercariae were fixed in 2.5% glutaraldehyde and processed for SEM. The glutaraldehyde-fixed cercariae and metacercariae (excysted) were washed with 0.1 M phosphate buffer (pH 7.4) several times, dehydrated through graded series of ethanol (50%, 70%, 80%, 90%, 95%, and 100%), and freeze-dried in a freeze dryer (Edwards, Crawley, UK). The samples were gold-coated with an Eiko-3 ion coater (Hitachi, Tokyo, Japan) and observed by a DS-130 SEM (ISI, Tokyo, Japan) under an accelerating voltage of 15 kV [
13].
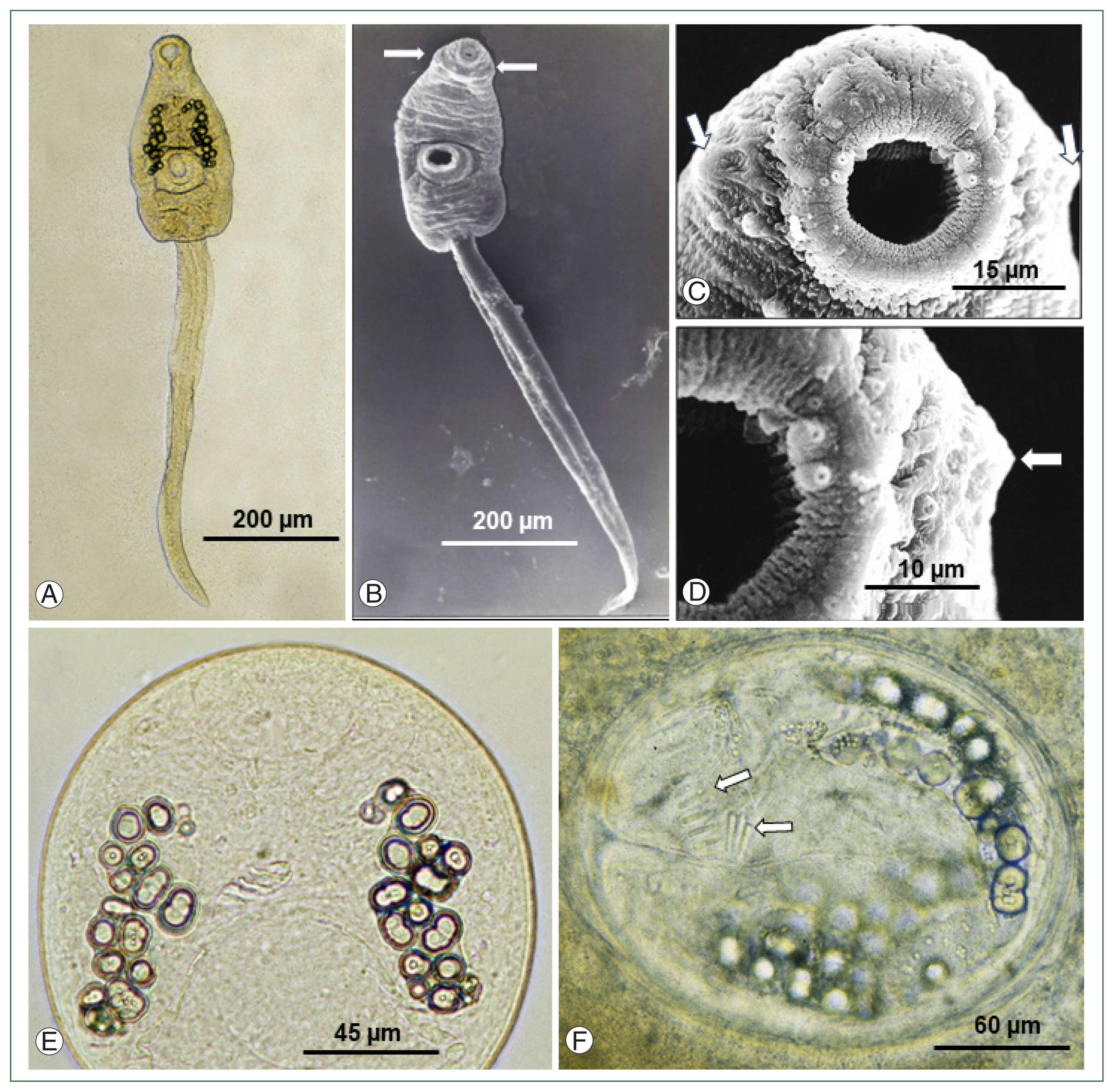
Under LM, the cercariae comprised of a spindle-shaped body (equipped with oral, ventral suckers, head collar, excretory granules, etc.) and a long, slender, and rod-like tail with a sharp attenuated, and pointed end (
Fig. 1A). Approximately 18–25 small round excretory granules were observed on each side of the anterior portion to the ventral sucker; however, collar spines were not recognizable (
Fig. 1A). SEM observations confirmed the presence of the head collar around the oral sucker of the cercariae, although its developmental status was weak and without the appearance of collar spines (
Fig. 1B). During magnifications of the head part of the cercariae (
Fig. 1C, D), the presence of the head collar was recognizable; however, collar spines were still not visible. The development of collar spines was not recognized even in the metacercariae of 3, 6, 12 (
Fig. 1E;
Supplementary Fig. S1), 15, and 20 h of age. The development of collar spines was confirmed only in the metacercariae of >24 h of age; at 24 h (in 10 of 20 metacercariae; 50%), 26 h (in all 20 metacercariae; 100%), and 30 h (in all 20 metacercariae, 100%,
Fig. 1F). A total of 27 collar spines with 4 ventral corner spines on each side and 23 dorsal spines in double row were counted in fully mature metacercariae older than 24 h after infection in tadpoles.
The results revealed that the head collar of I. hortensis developed early in the cercarial stage although the degree of development was weak. Its presence was difficult to observe under LM but easily located under SEM. The development of collar spines was not recognized while the parasite was in the cercarial stage but was evident in the metacercarial stage after 24 h of infection. The results indicated that finding and counting the number of collar spines in the cercariae of I. hortensis shed from lymnaeid snails (Lymnaea spp. or Radix spp.) is not a feasible method for its familial (echinostome vs. fasciolid cercariae) and specific diagnosis (differentiation from other echinostome species).
Schell [
14] mentioned that the number and arrangement of collar spines on the head collar are important morphological features for speciation of the family Echinostomatidae (=echinostome) adults as well as larvae, including the cercariae and metacercariae. However, the presence or absence of collar spines in the cercarial stage differs among the species of echinostome flukes [
15]. For example, collar spines were not developed in the cercarial stages of
Echinochasmus species (20~24- or 30~34-collar-spined), including
E. elongatus,
E. grandis,
E. japonicus,
E. perfoliatus,
E. milvi,
E. rugosus, and
E. tobi [
15]. However, in other species of
Echinochasmus, such as
E. redioduplicatus, collar spines were incompletely developed in the cercarial stage, with 3 pairs (6 among the total 32 spines) on the ventral side of the oral sucker [
15]. Similarly, the cercariae of
Acanthoparyphium sp. (22- or 23-collar-spined) of Yamaguti, 1934 revealed incompletely developed collar spines [
15]. Although, in other genera of the Echinostomatidae, for example,
Echinostoma spp. (31~55-collar-spined), including
E. revolutum,
E. cinetorchis,
E. gotoi,
E. macrorchis,
E. caproni, and
E. trivolvis, collar spines were already developed in their cercarial stage [
14–
17]. Similarly, species of
Echinoparyphium (29~45-collar-spined), including
E. recurvatum and
Echinoparyphium sp., revealed already developed collar spines in their cercarial stage [
15,
17]. Thus, it can be concluded that the appearance of collar spines in larval stages, especially in the cercarial stage varies depending on the species of echinostomes.
The genus
Isthmiophora is known to consist of at least 7 different species, namely,
I. hortensis,
I. melis (Schrank, 1788) Lühe, 1909 (syn.
Echinostoma melis),
I. inermis (Fuhrmann, 1904) Kostadinova and Gibson, 2002 (syn.
Euparyphium inerme),
I. beaveri (Beaver, 1941) Kostadinova and Gibson, 2002 (syn.
Euparyphium beaveri,
Euparyphium melis),
I. lukjanovi (Chertkova, 1971) Kostadinova and Gibson, 2002 (syn.
Euparyphium lukjanovi),
I. citellicola (Kadenatsii in Skryabin and Bashkirova, 1956) Kostadinova and Gibson, 2002 (syn.
Echinostoma citellicola), and
I. hominis (Kifune and Takao, 1973) Kostadinova, 2005 (syn.
Psilorchis hominis) [
18,
19]. In Korea, the existence of
I. hortensis [
1–
3],
I. inermis (differs from
I. hortensis in having a slender body, elongated testes, and short anterior limit of vitellaria) [
20], and an unidentified 27-collar-spined echinostome [
21] (suggested to be
I. hortensis) has been reported. The species involved in our experiment was confirmed to be
I. hortensis by obtaining adult flukes from experimental rats infected with metacercariae (data not shown).
Among the
Isthmiophora spp., studies on larval stages have been undertaken in 3 species, including
I. hortensis [
5,
8–
11],
I. melis [
22], and
I. beaveri [
23]. In the case of
I. hortensis, as mentioned above, the presence or absence of collar spines on the head collar in the cercarial stage has been controversial [
8–
11]. Regarding
I. melis, 27 collar spines were well developed in the cercarial stage, including 4 corner spines on each side and 19 dorsal and lateral spines in the oral and adoral rows, respectively [
22]. In
I. beaveri, collar spines were easily observed, with 4 angle spines on each side, 6 laterals on each side in a single row, and 7 dorsals in an alternative row [
23].
Compared with the full development of collar spines in the cercarial stage of
I. melis and
I. beaveri, it is difficult to explain why the collar spines of
I. hortensis did not appear in the cercarial stage in our experimental setting. A few possible explanations include, firstly, a specific character for
I. hortensis (delayed development of collar spines) that is similar to the previously mentioned species of
Echinochasmus. If so, the results of the 3 previous studies on the life cycle of
I. hortensis [
8–
10] are difficult to explain. Second, the 30-day-old cercariae of
I. hortensis used in our study might have been immature cercariae; if we had examined 40-, 50-, or 60-day-old cercariae, they might have been equipped with visible collar spines. However, we were successful in the infection of experimental tadpoles with the 30-day-old cercariae in which they grew to be metacercariae; these metacercariae (older than 24 h of infection) revealed collar spines and were able to successfully infect experimental rats. Thus, it can be assured that the 30-day-old cercariae in the snail were fully mature ones. Further experiments are needed to elucidate whether cercariae older than 40–60 days from laboratory-infected snails and/or naturally infected snails (age of infection unknown) would reveal collar spines on the head collar.
Fig. 1Development of collar spines in larval stages of Isthmiophora hortensis. (A) A cercaria shed from a Lymnaea pervia snail experimentally exposed to miracidia hatched from eggs liberated from adult flukes obtained from an experimental rat. The head collar is faintly recognizable. Oral and ventral suckers, excretory granules, and a tail are seen. (B) Scanning electron microscopy (SEM) view of a cercaria shed from an L. pervia snail. The head collar is easily recognized (arrows). (C) SEM view of the head collar (arrows) around the oral sucker of a cercaria. Sensory papillae are seen on and around the oral sucker, but no collar spines are observed. (D) A magnified SEM view of (C). The head collar (arrow), oral sucker, and sensory papillae are seen, but no collar spines are present. (E) A 12-h-old metacercaria in the gill of a tadpole experimentally infected with the cercariae showing no collar spines yet. (F) A 30-h-old metacercaria from the gill of an experimentally infected tadpole showing well-developed collar spines (arrows).
Notes
-
The authors declare that they have no conflict of interest related to this study.
-
Conceptualization: Sohn WM, Chai JY
Project administration: Chai JY
Funding acquisition: Shin EH
Methodology: Sohn WM, Jung WJ
Investigation: Sohn WM, Jung WJ
Data curation: Sohn WM, Jung WJ
Formal analysis: Sohn WM, Chai JY
Visualization: Sohn WM, Chai JY
Validation: Sohn WM, Chai JY
Supervision: Sohn WM, Shin EH, Chai JY
Writing-original draft: Shon WM, Shin EH, Chai JY
Writing-review and editing: Shin EH, Chai JY
Supplementary Information
Supplementary Fig. S1.
A 12-h-old metacercaria of Isthmiophora hortensis obtained from an experimentally infected tadpole showing the presence of a head collar (arrows) but no collar spines yet. A dorsolateral view. OS, oral sucker. Scale bar = 10 μm.
Acknowledgment
We are grateful to all members of our laboratory, Department of Tropical Medicine and Parasitology, Seoul National University College of Medicine, Seoul, Korea who helped us with this experimental study.
References
- 1. Chai JY, Jung BK. General overview of the current status of human foodborne trematodiasis. Parasitology 2023;149(10):1262-1285. https://doi.org/10.1017/S0031182022000725
- 2. Chai JY. Human Intestinal Fluke: from Discovery to Treatment and Control. Springer; Dordrecht, The Netherlands. 2019, pp 1-549 https://doi.org/10.1007/978-94-024-1704-3
- 3. Lee SK, Chung NS, Ko IH, Sohn WM, Hong ST, et al. An epidemiological survey of Echinostoma hortense infection in Chongsong-gun, Kyongbuk Province. Parasites Hosts Dis 26(3):199-206. (in Korean). https://doi.org/10.3347/kjp.1988.26.3.199
- 4. Son WY, Huh S, Lee SU, Woo HC, Hong SJ. Intestinal trematode infections in the villagers in Koje-myon, Kochang-gun, Kyongsangnam-do, Korea. Parasites Hosts Dis 1994;32(3):149-155. https://doi.org/10.3347/kjp.1994.32.3.149
- 5. Ahn YK, Kang HS. Development and cercarial shedding of Echinostoma hortense in the snail host, Radix auricularia coreana. J Wonju Med Coll 1988;1(1):137-152. (in Korean).
- 6. Sohn WM, Na BK, Cho SH, Ju JW. Infection status of Isthmiophora hortensis metacercariae in dark sleepers, Odontobutis species, from some water systems of the Republic of Korea. Parasites Hosts Dis 2018;56(6):633-637. https://doi.org/10.3347/kjp.2018.56.6.633
- 7. Chai JY, Lee SH. Food-borne intestinal trematode infections in the Republic of Korea. Parasitol Int 2002;51:129-154. https://doi.org/10.1016/S1383-5769(02)00008-9
- 8. Ono S. The life history of Echinostoma campi n. sp. found in the vicinity of Mukden, with special reference to the second intermediate host. Dobutsugaku Zasshi 1930;42:7-16. (in Japanese).
- 9. Ono S. Studies on the trematodes invading Lymnaea snails as the first intermediate hosts found in the vicinity of Mukden (I). Nippon Juigaku Zasshi 1935;14:25-35. (in Japanese).
- 10. Yamaguti S. Zur Entwicklungsgeschichte von Echinostoma hortense Asada, 1926, mit besonderer Berücksichtigung der Struktur der Cercarie. Z Parasitenkd 1941;12(2):273-276. https://doi.org/10.1007/BF02121244
- 11. Okamoto F. Experimental studies on trematodes in the south of Hokkaido (I). Jpn J Parasitol 1954;3(3–4):216-220. (in Japanese).
- 12. Lee SH, Hwang SW, Sohn WM, Kho WG, Hong ST, et al. Experimental life history of Echinostoma hortense. Parasites Hosts Dis 1991;29(2):161-172. https://doi.org/10.3347/kjp.1991.29.2.161
- 13. Jung WJ. Studies on tegumental ultrastructures of the cercariae and metacercariae of Echinostoma hortense and appearance of their collar spines. M. Sc. Thesis. Seoul National University Graduate School; 1990.
- 14. Schell SC. How to Know the Trematodes. WM.C. Brown Com. Pub; Dubuque, Iowa. . 1970, pp 18-35.
- 15. Ito J. A monograph of cercariae in Japan and adjacent territories. Progr Med Parasitol Japan 1964;1:395-550.
- 16. Fried B, Kanev I, Reddy A. Studies on collar spines of echinostomatid trematodes. Parasitol Res 2009;105:605-608. https://doi.org/10.1007/s00436-009-1519-5
- 17. Fried B, Frazer BA, Kanev I. Comparative observations on cercariae and metacercariae of Echinostoma trivolvis and Echinoparymphium sp. J Parasitol 1998;84:623-626. https://doi.org/10.2307/3284737
- 18. Kostadinova A, Gibson DI. Isthmiophora Lühe, 1909 and Euparyphium Dietz, 1909 (Digenea: Echinostomatidae) re-defined, with comments on their nominal species. Syst Parasitol 2002;52:205-217. https://doi.org/10.1023/A:1015789703396
- 19. Kostadinova A. Family Psilostomidae Looss 1900. In Jones A, Bray RA, Gibson DI eds, Key to the Trematoda. 2:Natural History Museum; London, UK. . 2005, pp 99-118.
- 20. Choe S, Na KJ, Kim Y, Jeong DH, Yang JJ, et al. Infections of two Isthmiophora species (Digenea: Echinostomatidae) in wild mammals from Republic of Korea with their morphological descriptions. Parasites Hosts Dis 2019;57(6):647-656. https://doi.org/10.3347/kjp.2019.57.6.647
- 21. Sohn WM, Na BK. Infections with digenetic trematode metacercariae in freshwater fishes from two visiting sites of migratory birds in Gyongsangnam-do, Republic of Korea. Parasites Hosts Dis 2019;57(3):273-281. https://doi.org/10.3347/kjp.2019.57.3.273
- 22. Grabda-Kazubska B, Laskowski Z. On the morphology and chaetotaxy of rediae and cercariae of Isthmiophora meliis (Schrank, 1788) (Trematoda, Echinostomatidae). Acta Parasitol 1996;41(1):7-12.
- 23. Beaver PC. Studies on the life history of Euparyphium melis (Trematoda: Echinostomatidae). J Parasitol 1941;27(1):35-44. https://doi.org/10.2307/3272884