Abstract
The surface ultrastructure of Acanthotrema felis (Trematoda: Heterophyidae) adults, recovered from a kitten experimentally infected with the metacercariae, was observed using a scanning electron microscope. The worm was leaf-like, ventrally concave and covered with scale-like multi-pointed tegumental spines. The spines on the anterior surface were short but broad, and had 10-12 pointed tips. The cytoplasmic processes protruded around the spines, like pockets for the spines. The ventrogenital opening was crescent, or kidney-shaped, and had protuberances with minute spines on its surrounding tegument. The spines on the posterior surface were long, but narrow, with 6-8 pointed tips. The cytoplasmic processes on this tegument were ridge-like, and elevated along the row of the spines. The surface ultrastructure of A. felis is generally similar to that of other heterophyid flukes, but some features are characteristic, and may be of taxonomic and bio-ecological significance.
-
Key words: Acanthotrema felis, heterophyid, cat, surface ultrastructure
Acanthotrema felis (Trematoda: Heterophyidae) is a minute intestinal fluke of cats in the Republic of Korea (
Sohn et al., 2003). For the genus
Acanthotrema, more than 7 species, including the type,
A. acanthotrema, are known (
Sohn et al., 2003). They are morphologically very similar to those of the genus
Stictodora (
Sohn et al., 2003).
The surface ultrastructure of trematodes gives a clue to the understanding of their morphological and taxonomic characteristics. Studies on the surface ultrastructure of Heterophyidae have been performed in species of the genera
Centrocestus (
Srisawangwong et al., 1997;
Woo et al., 1998),
Cryptocotyle (
Køie, 1977),
Haplorchis (
Fujino et al., 1989),
Heterophyes (Taraschewski, 1984;
Chai et al., 1992;
Uga et al., 1998),
Heterophyopsis (
Hong et al., 1991),
Metagonimus (
Lee et al., 1984;
Fujino et al., 1989;
Chai et al., 1998,
2000),
Pygidiopsis (
Køie, 1992) and
Stictodora (
Sohn, 2000). However, the surface ultrastructure of
Acanthotrema species has not been reported. In the present study, in order to distinguish the characteristic features of the surface ultrastructure of
A. felis, the adult flukes recovered from an experimental kitten fed the metacercariae were observed by scanning electron microscopy.
The metacercariae of A. felis were isolated from a species of the goby, Acanthogobius flavimanus, purchased at a local market in Shinan-gun, Jeonranam-do, Korea, using the artificial digestion technique. The metacercariae were fed orally to a kitten through a gavage needle. The adult flukes were recovered from the kitten at day 7 post-infection (PI). For the scanning electron microscopy, the collected worms were washed twice with physiological saline and three times with 0.1 M cacodylate buffer (pH = 7.2). They were fixed with 2.5% glutaraldehyde at 4℃, and post-fixed with 1% osmium tetroxide for 2 hr at room temperature. After further washing with 0.1 M cacodylate buffer (pH 7.2), the specimens were dehydrated in a graded series of ethanol (50%, 70%, 80%, 90%, 95% and absolute), dried in an ABT (model CP-5A) critical point dryer, and mounted on stubs. They were coated with gold using an ion sputtering coater (Bio-Rad, E 5300) and observed by scanning electron microscopy (ISI DS-130C, Japan) at an accelerating voltage of 10 kV.
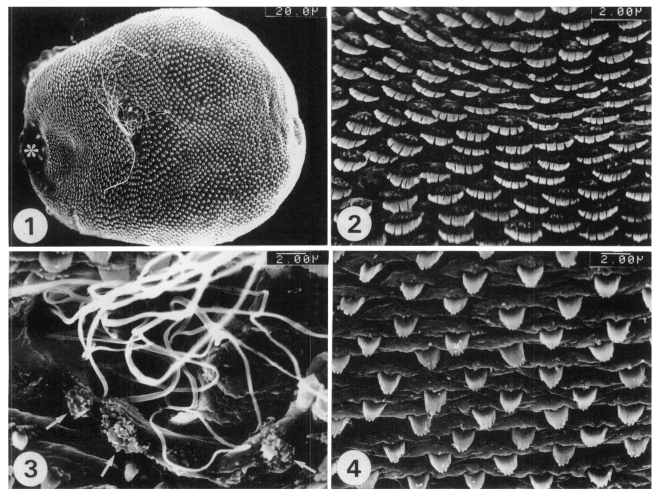
The body of the worms was leaf-like, ventrally concave and covered with scale-like multi-pointed tegumental spines. The ventrogenital opening was located in the anterior 1/3
rd of the body, and slightly deviated from the median line (
Fig. 1). The tegumental spines on the antero-ventral surface of the ventrogenital opening were short, but broad, with 10-12 pointed tips. The cytoplasmic processes protruded around the tegumental spines, like pockets for the spines (
Fig. 2). The ventrogenital opening was crescent, or kidney-shaped, and had protuberances with minute spines on its sur-rounding tegument; the sperm were discharged from the genital pore (
Fig. 3). The tegumental spines on the postero-ventral surface of the ventrogenital opening were long, but narrow, with 6-8 pointed tips. The cytoplasmic processes on this tegument were ridge-like, and elevated along the row of tegumental spines (
Fig. 4).
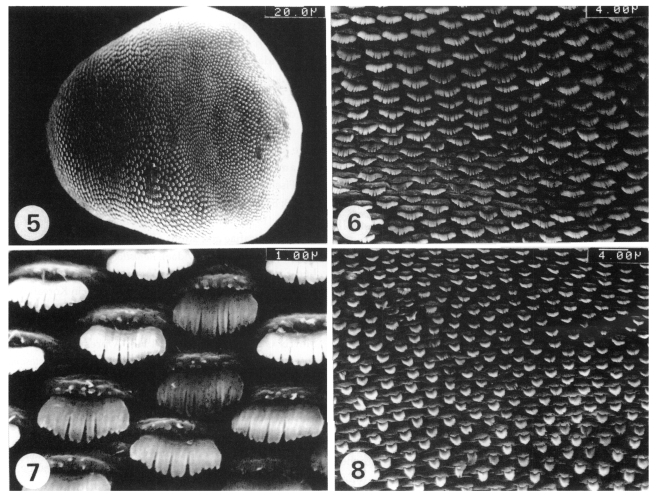
The whole dorsal surface was covered with scale-like multi-pointed tegumental spines. The spines were densely and regularly distributed over the whole dorsal surface (
Fig. 5). The tegumental spines and cytoplasmic processes on the antero-dorsal surface were similar to those on the antero-ventral surface (
Figs. 6 and
7). The tegumental spines on the postero-dorsal surface were long, but narrow, with 6-8 pointed tips (
Fig. 8). The cyto-plasmic processes on this tegument were also ridge-like (
Fig. 8).
With regard to the surface ultrastructure of Acanthotrema, no previous reports have been available. The results of this study show that the tegumental ultrastructure of A. felis is generally similar to that of other heterophyids in terms of the tegumental spines and cytoplasmic processes, and in the distribution patterns of the tegumental spines. However, the tegumental surface of the ventrogenital sac, which showed protuberances with minute spines, but without sensory papillae, is suggested to be characteristic to A. felis.
The multi-pointed (saw tooth-shaped) tegumental spines seem to be a common characteristic feature of the family Heterophyidae. In this study, the tegumental spines of
A. felis were digitated with 9-12 and 6-8 points. Those of
H. nocens were, in most cases, 12-17 pointed (
Chai et al., 1992). Similar findings have been observed in other heterophyid flukes (
Køie, 1977,
1992;
Lee et al., 1984; Taraschewski, 1984;
Fujino et al., 1989;
Hong et al., 1991;
Chai et al., 1992,
1998,
2000;
Srisawangwong et al., 1997;
Uga et al., 1998;
Woo et al., 1998).
The degree of digitation at the tip of the spines and its changing patterns according to the maturation of worms seem to vary by species of flukes. For the case of
H. continua, the number of digitations in the tegumental spines of the anterior body increased from 10-14 to 15-17 as the worm matured from a metacercaria to the adult fluke (
Hong et al., 1991). Similar findings have been reported in other heterophyid flukes (
Chai et al., 1998,
2000). However, information on the surface ultrastructure of the metacercariae and juvenile worms of
A. felis are, at present, unavailable, and the changing patterns of the tegumental spines according to the maturation of worms are unknown.
In A. felis, the digitations of the tegumental spines decreased toward the posterior end of the body. The higher digitation of tegumental spines between the oral sucker and ventrogenital opening of A. felis may be related to the abrasion of the host intestinal epithelium for their anchorage and feeding. It is generally known that the two suckers of intestinal trematodes play an important role in their protection from being expelled due to the peristalsis of the host intestines. In A. felis, however, the oral sucker is small, probably not powerful, and the ventral sucker is also small, and incorporated into a ventrogenital sac, which appears to be far from powerful for an adhesive organ.
The functions of the sensory papillae have been speculated as being those of tango- or chemo-receptors, as inferred from their shapes and positions in the body, and from the results of transmission electron microscopic studies (
Morris and Threadgold, 1967). The sensory papillae probably play an important role in the physiological and metabolic functions of the trematodes in their ecological niche. Interestingly, however, no sensory papillae were observed on the tegumental surface of
A. felis. Whether this is a unique and characteristic feature among the flukes of the genus
Acanthotrema should be clarified by further studies.
References
- 1. Chai JY, Chung HL, Choi MH, Sohn WM, Hong SJ, Lee SH. Surface ultrastructure of Heterophyes nocens (Trematoda: Heterophyidae). Korean J Parasitol 1992;30:75-82.
- 2. Chai JY, Guk SM, Han ET, et al. Surface ultrastructure of Metagonimus takahashii metacercariae and adults. Korean J Parasitol 2000;38:9-15.
- 3. Chai JY, Kang YJ, Choi SY, Guk SM, Yu JR, Lee SH. Surface ultrastructure of Metagonimus miyatai metacercariae and adults. Korean J Parasitol 1998;36:217-225.
- 4. Fujino T, Higo H, Ishii Y, Saito S, Chen ER. Comparative studies on two similar species of Haplorchis and Metagonimus (Trematoda: Heterophyidae) - surface ultrastructure of adults and eggs. Proc Helminthol Soc Wash 1989;56:35-41.
- 5. Hong SJ, Chai JY, Lee SH. Surface ultrastructure of the developmental stage of Heterophyopsis continua (Trenatoda: Heterophyidae). J Parasitol 1991;77:613-620.
- 6. Køie M. Stereoscan studies of cercariae, metacercariae and adults of Cryptocotyle lingua (Creplin, 1825) Fischoeder, 1903 (Trematoda: Heterophyidae). J Parasitol 1977;63:835-839.
- 7. Køie M. Scanning electron microscopy of cercariae, metacercariae and adults of Pygidiopsis ardeae Køie, 1990 (Digenea: Heterophyidae). Parasitol Res 1992;78:469-474.
- 8. Lee SH, Seo BS, Chai JY, Hong SJ. Study on Metagonimus yokogawai (Katsurada, 1912) in Korean. VII. Electron microscopic observation on the tegumental structure. Korean J Parasitol 1984;22:1-10. (in Korean).
- 9. Morris GP, Threadgold LT. A presumed sensory structure associated with the tegument of Schistosoma mansoni. J Parasitol 1967;53:537-539.
- 10. Sohn WM. Surface ultrastructures of Stictodora fuscatum (Trematoda: Heterophyidae). Korean J Elect Microscopy 2000;30:205-212.
- 11. Sohn WM, Han ET, Chai JY. Acanthotrema felis n. sp. (Digenea: Heterophyidae) from the small intestine ofstray cats in the Republic of Korea. J Parasitol 2003;89:154-158.
- 12. Srisawangwong T, Pinlaor S, Kanla P, Sithithaworn P. Centrocestus formosanus: surface morphology of metacercaria, adult and egg. J Helminthol 1997;71:345-350.
- 13. Uga S, Morimoto M, Saito T, Rai SK. Surface ultrastructure of Heterophyes heterophyes (Trematoda: Heterophyidae) collected from a man. J Helminthol Soc Wash 1998;65:119-122.
- 14. Woo HC, Seo MD, Hong SJ. Surface ultrastructure of juvenile and adult stages of Centrocestus armatus. J Helminthol 1998;72:215-219.
Figs. 1-4
Fig. 1. Ventral view of a whole adult worm of Acanthotrema felis, recovered from the intestine of an experimentally infected cat, showing the oral sucker (*) and ventrogenital opening (arrow). Fig. 2. The tegument on the antero-ventral surface of the ventrogenital opening, on which the spines are short, but broad, with 10-12 pointed tips. Fig. 3. The ventrogenital opening showing several protuberances with minute spines (arrows), and white filament-like sperm discharged from the genital pore. Fig. 4. The tegument on the postero-ventral surface of the ventrogenital opening, where the spines are long, but narrow, with 6-8 pointed tips.

Figs. 5-8
Fig. 5. Dorsal view of a whole adult worm, where the surface is densely covered with the tegumental spines. Fig. 6. The tegument on the antero-dorsal surface, where the spines are short, but broad, with 10-12 pointed tips. Fig. 7. A higher magnification of a part of Fig. 6. Fig. 8. The tegument on the postero-dorsal surface, where the spines are long, but narrow, with 6-8 pointed tips.

Citations
Citations to this article as recorded by

- Body Surface Ultrastructure as a Main Morphological Criterion for Distinguishing Adult Trematode Metagonimus suifunensis
Polina Shumenko, Yulia Tatonova, Mikhail Shchelkanov
Biology.2024; 13(11): 942. CrossRef - Anthelmintic potential of sulphonamides and Cucurbita pepo seeds extract on Heterophyes heterophyes experimentally infected mice
Dalia S. Ashour, Fetouh A. Deyab, Kamal F. Eliwa, Samy I. El-Kowrany
Journal of Parasitic Diseases.2023; 47(4): 697. CrossRef - Surface ultrastructure of the adult and juvenile stages of the trematode Astiotrema impletum (Looss, 1899) Looss 1900 (incertae sedis) from the Nile puffer, Tetraodon lineatus Linnaeus, 1758
S. G. Abd El-Kareem, M. H. Ibraheem
Helminthologia.2021; 58(2): 188. CrossRef - REDESCRIPTION AND SURFACE ULTRASTRUCTURE OF PYGIDIOPSIS MACROSTOMUM (DIGENEA: HETEROPHYIDAE)
S. B E. Simões, H. S. Barbosa, C. P. Santos
Journal of Parasitology.2005; 91(4): 931. CrossRef