Abstract
The effects of in vitro culture methods on morphological development and infectivity of Strongyloides venezuelensis filariform larvae (L3) to rats were investigated. A significantly higher body length was observed in L3 from filter paper culture (597.3 ± 32.2 µm) than those in fecal (509.9 ± 35.0 µm) and nutrient broth culture (503.3 ± 31.0 µm) (P<0.05). Larval infectivity was assessed by exposing rats to 1,000 L3 from each culture and worms were recovered from the lungs and small intestines. Recovery rate of these worms did not show any significant difference. A significantly greater body length of adults was recorded in those corresponding to the L3 harvested from filter paper (2,777.5 ± 204.4 µm) and nutrient broth culture (2,732.5 ± 169.8 µm) than those corresponding to the L3 obtained from fecal culture (2,600.5 ± 172.4 µm) (P<0.05). Although worm fecundity and EPG counts differed among culture methods but worm burdens and course of infection did not. These findings suggest that the methods of cultures have a significant effect on the morphological development of the larvae to the L3 stage, but do not influence the infectivity to rats.
-
Key words: Strongyloides venezuelensis, in vitro culture, filariform larvae, morphological development, infectivity
INTRODUCTION
Recently we have demonstrated that a chemical medium can be used as a substitute for infected-rat feces for harvesting the third-stage filariform larvae (L
3) of
Strongyloides venezuelensis from eggs in a polyvinyl culture bag (
Baek et al., 1998a). The egg hatch rate and larval metamorphosis to the L
3 stage are greatly influenced by incubation temperatures, concentration of culture medium and numbers of eggs inoculated in the culture bags. We have also showed that larval development rate to the L
3 stage is significantly different according to the in vitro culture methods. In a subsequent experiment, the viability of
S. venezuelensis in different stages maintained in vitro has been assessed (
Baek et al., 1998b). Eggs, L
3 and adults of
S. venezuelensis obtained from experimentally infected rats can survive for an extended period in various culture media at different temperatures. The development of intestinal nematode from the free-living stage (e.g., L
3 stage) to the parasitic adult stage depends on several factors related to host (e.g., species, strain, age, sex and resistance) and parasite species (e.g., strain, infective dose and culture age of L
3) (
Solomon and Haley, 1966;
Carter and Wilson, 1989;
Sato and Toma, 1990;
Khan et al., 1993). Taira et al. (
1994) have demonstrated that the inoculation routes of
S. venezuelensis L
3 significantly influence the worm burdens. Additionally, studies on morphological development and infectivity of free-living stage (e.g., L
3) and parasitic adult nematodes are considered useful in analysing the mode of action of anthelmintics and resistance thereto (
Martin, 1997). However, there has been, so far, no report on the comparative analysis of morphological development and infectivity of L
3 stage and parasitic adults of
Strongyloides species according to the methods of in vitro cultures. Therefore this study was attempted to report on the effects of in vitro culture methods including currently established nutrient broth culture (
Baek et al., 1998a) on morphological development of L
3 and their infectivity to rats; recovery rate of migrating stage larvae (MSL
3) and adults from the lungs and the small intestines, respectively, morphological development of the corresponding adults, worm burdens in the small intestines, fecundity, egg outputs and persistence of infection.
MATERIALS AND METHODS
Animal and parasite
Eight-week-old inbred male Fischer (F344) rats, weighed about 150 g, supplied by the Korean Chemical Research Institute were used in this study. A total of 120 rats was used, which composed of three experimental groups with 40 rats each. Each group was further divided into eight subgroups with five rats. Laboratory maintenance of
S. venezuelensis has been previously described (
Baek et al., 1998a).
The L
3 were obtained from three different in vitro culture methods: (i) nutrient broth culture (NBC) using isolated eggs in 0.12% nutrient broth, (ii) fecal culture (FC) consisting of isolated eggs and feces (2%) of uninfected-rats, and (iii) filter paper culture (FPC) comprising infected rats' feces smeared on a large filter paper (15 × 30 cm), which is placed in a beaker (1,000 ml) containing a small quantity of water (
Baek et al., 1998a). The larvae were collected from each culture separately and washed five times in sterile saline (SS). In order to determine the effects of culture methods on morphological development of larvae, fifty L
3 harvested from each culture were measured for body length, esophagus, tail length, and body width at esophageal-intestinal (E-I) junction.
Approximately, 1,000 L3 were concentrated in 250 µl SS, and used as an infective dose for each rat. The subgroups of rats were infected subcutaneously with 1,000 L3 at the back of the neck under light anesthesia with ether.
Infectivity of L
3 harvested from NBC, FC and FPC was assessed by recovering the MSL
3 from the lungs and the adult worms from the small intestines of rats after infection with 1,000 L
3 obtained from the respective cultures (
Wertheim, 1970b;
Korenaga et al., 1991). Briefly, the MSL
3 were collected and counted from the lungs of rats on day 3 post-infection (PI). The lungs were minced into small pieces and incubated in SS at 37℃ for 4 hr in a Baermann device. The MSL
3 falling down to the bottom of the tube were counted under a dissecting microscope. The adults were recovered from the small intestine of rats which were killed at different time intervals. The worms attached to the mucosa were also counted under a dissecting microscope.
Morphological development of the adults recovered on day 7 PI from each experimental group was assessed by measuring the whole body length, esophagus, tail length, and the distance from mouth to vulva. Body width at E-I junction, vulva and anus were also measured. Worm fecundity in terms of eggs in utero per worm was examined immediately after recovery. Intensity of infection was monitored by examining daily egg outputs (EPG) and worm burdens in the small intestines of five rats per group at different time intervals. Freshly passed feces from five rats per group were collected in a 50 ml glass beaker and EPG was counted by the method of Sato and Toma (
1990).
Data were analysed by Welch's t-test and the significance was determined at 5% level.
RESULTS
Morphological development of filariform larvae
Morphological development of the L
3 obtained from each in vitro culture was described in
Table 1. The L
3 harvested from FPC (597.3±32.2 µm) showed a significant increase of body length (P<0.05), whereas that obtained from FC (509.9±35.0) and NBC (503.3±31.0) was almost similar in size.
Recovery rate of the MSL
3 from the lungs on day 3 PI was 10.9%, 10.5% and 9.4% of the infective dose prepared from FPC, NBC and FC, respectively (
Fig. 1). Recovery rate of adults was 17.4%, 16.7% and 15.3% of the infective dose obtained from FPC, NBC and FC, respectively on day 7 PI (
Fig. 1). No significant differences were observed in recovery rate of both MSL
3 and adults among culture methods of L
3 for experimental infection (P<0.05).
Morphological development of the adults recovered on day 7 PI was compared among three kinds of culture methods (
Table 2). The body length of adults was significantly increased in rats infected with the L
3 obtained from FPC (2,777.5±204.4 µm) and NBC (2,732.5±169.8) as compared with those recovered from rats infected with the L
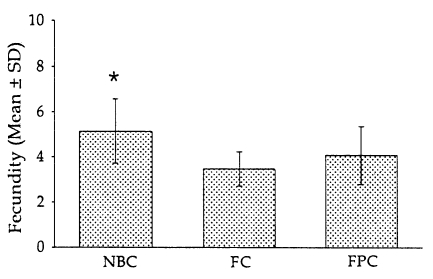
3 from FC (2,600.5±172.4) (P<0.05). The body width at E-I junction, vulva and anus was almost equal in measurement. Fecundity was significantly high in female worms corresponding to the L
3 obtained from NBC (5.1±1.4) than in those from FC (3.5±0.8) (P<0.05), but not in those from FPC (4.1±1.3) (
Fig. 2).
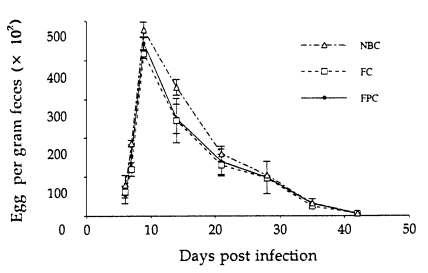
Regardless of the methods of in vitro cultures, eggs appeared first in the feces on day 6 PI, and EPG counts peaked on day 9 PI, and then gradually declined to zero by day 45 PI (
Fig. 3). However, significantly higher EPG counts were recorded in rats infected with the L
3 harvested from NBC than in those from FC (P<0.05), but not in those from FPC. Worm burdens in all groups peaked on day 7 PI, gradually declined and reached the zero by day 49 PI (
Table 3), showing no significant difference according to the culture methods.
DISCUSSION
Strongyloides venezuelensis, a natural parasite of the rat (
Brumpt, 1934), initiate a parasitic life cycle by the L
3, which penetrate host's skin, migrate to the lungs and then travel to the small intestine where they eventually develop to the adult worms. It has been considered as a suitable model for the study of host-parasite interaction of strongyloidiasis since the parasite can be easily passaged in the laboratory and large numbers of eggs and larvae are obtained from rats exposed to 10,000-30,000 L
3 (
Taira et al., 1994). In addition, this nematode does not cause sudden death even in massive infection in rats (
Taira et al., 1995) as compared with another closely related species,
Strongyloides ratti, which can cause sudden death in rats and Mongolian gerbils. The morphology of L
3 obtained from different conventional in vitro cultures as well as those of the in vivo derived adult worms have been reported previously (
Little, 1966;
Hasegawa et al., 1988;
Taira et al., 1994). After primary infection with
S. venezuelensis, the subsequent development to the parasitic adult stage in a single and/or concurrent infections with
S. ratti, pulmonary and intestinal worm burdens, kinetics of egg outputs, persistence of infection and immunological responses have been reported in rats (
Werthiem, 1970a,
1970b;
Carter and Wilson, 1989), mice (
Sato and Toma, 1990), Mongolian gerbils (
Tsuji et al., 1993) and Syrian golden hamsters (
Shi et al., 1994). In the present study, we have demonstrated that in vitro culture methods have significant effects on morphological development to the L
3 stage, however, they do not influence the infectivity of the larvae to rats.
A significantly greater body length but not body width of the L
3 were observed in FPC than those in NBC and FC, which also reflected on subsequent morphological development of the corresponding adult worms with an increased body length but not body width. The reason for such differences in morphological dimensions of the L
3 and of corresponding adults according to the culture methods is not clear. It may be explained that an increased body length of the L
3 harvested from FPC is due to the use of infected-rat feces directly in the culture device without isolating and repeatedly washing the eggs in SS as was done in NBC and FC (
Baek et al., 1998a). Thus the larval microenvironment was not physically interfered in FPC and the larvae gain optimum morphological development. However, in the previous report, we have shown that the saturated salt solution used for the isolation of eggs does not affect the egg hatch rate, and yields of the L
3 are higher in NBC as compared to FPC (
Baek et al., 1998a). The reason for higher morphological development of adult worms corresponding to FPC is difficult to explain. Morphological dimensions with respect to body length of the L
3 recorded in NBC and FC except for FPC were observed smaller than those described for
S. venezuelensis L
3 by several authors (
Little, 1966;
Wertheim, 1970a;
Hasegawa et al., 1988;
Taira et al., 1994). These findings suggest that growth and development of the L
3 are influenced not only by composition and/or methods of in vitro cultures but also by geographical and/or strain variations (
Hasegawa et al., 1988). Irrespective of the culture methods, morphological dimensions with respect to body length of adults recovered from Fischer (F344) rats on day 7 PI were greater than those reported by Little (
1966) and Taira et al. (
1994), but smaller than those reported by Wertheim (
1970a) and Hasegawa et al. (
1988). This variation may be due to the differences in rat strain, infective dose, parasite strain and stage of infection at which the worms were recovered.
Infectivity of the L
3 was not different according to the culture methods although recovery rate of the MSL
3 and adult worms was slightly higher in those corresponding to FPC than those corresponding to NBC and FC. However, infectivity has been known to be significantly influenced by the inoculation routes; higher infection rate was resulted from subcutaneous and percutaneous inoculation than from oral administration of L
3 (
Taira et al., 1994). The overall recovery rate of MSL
3 and adults in the present study are much higher than that observed in rats (
Taira et al., 1994), but much lower than those in mice (
Sato and Toma, 1990;
Khan et al., 1993;
Korenaga et al., 1995) and Mongolian gerbils (
Horii et al., 1993). In case of
Nippostrongylus brasiliensis infection, the development rate of adults was significantly higher with mouse strain than with rat strain of the parasite (
Solomon and Haley, 1966). Taken together, it is suggested that infectivity of the L
3 of intestinal nematodes to rats are significantly affected by the host species, routes of inoculation and parasite strains but not by in vitro culture methods.
A significantly higher fecundity of the parasitic females as well as higher EPG counts were observed in rats infected with the L
3 harvested from NBC than those from FC (P<0.05). The reason for such difference is not clear in this study. The fecundity of the parasitic females recovered from Fischer rats infected with the L
3 from NBC except FPC and FC are almost similar to that reported in mice by Sato and Toma (
1990). The time course of egg discharge, worm burdens and persistence of infection observed in Fischer rats were almost similar irrespective of the culture methods which further indicates that culture methods do not influence the infectivity of the L
3.
In our previous studies, we have determined suitable media and optimum temperature for in vitro culture of L
3 and maintenance of
S. venezuelensis in different stages, all of which are considered important for understanding the biology of the parasite (
Baek et al., 1998a,
1998b). The present results showed that larval morphological development is significantly influenced by the culture methods but their infectivity to the host is not. Our previous findings may contribute to the understanding and manipulating the life cycle for experimental studies with
Strongyloides species, and the present study provides a useful measure in analysing the mode of action of anthelmintics and resistance thereto.
Notes
-
This research work was supported in part by the Biosafety Research Institute, Chonbuk National University (1998).
References
- 1. Baek BK, Islam MK, Kim JH. Development of an in vitro culture method for harvesting the free-living infective larvae of Strongyloides venezuelensis. Korean J Parasitol 1998a;36:15-22.
- 2. Baek BK, Islam MK, Matsuda K. Viability of eggs, filariform larvae and adults of Strongyloides venezuelensis (Nematoda: Strongyloidea) maintained in vitro. Korean J Parasitol 1998b;36:99-107.
- 3. Brumpt E. Precis de Parasitologie. 1934, 6th ed. Paris, France. Masson et Cie. p 1042.
- 4. Carter KC, Wilson PAG. The course of infection in rats given small primary doses of Strongyloides ratti and S. venezuelensis. J Helminthol 1989;63:107-114.
- 5. Hasegawa H, Orido Y, Sato Y, Otsuru M. Strongyloides venezuelensis Brumpt, 1934 (Nematoda: Strongyloididae) collected from Rattus norvegicus in Naha, Okinawa, Japan. Jpn J Parasitol 1988;37:429-434.
- 6. Horii Y, Khan AI, Nawa Y. Persistent infection of Strongyloides venezuelensis and normal expulsion of Nippostrongylus brasiliensis in Mongolian gerbils, Meriones unguiculatus, with reference to the cellular responses in the intestinal mucosa. Parasite Immunol 1993;15:175-179.
- 7. Khan AI, Horii Y, Tiuria R, Sato Y, Nawa Y. Mucosal mast cells and the expulsive mechanisms of mice against Strongyloides venezuelensis. Int J Parasitol 1993;23:551-555.
- 8. Korenaga M, Hitoshi Y, Takatsu K, Tada I. Cross-resistance between Strongyloides venezuelensis and S. ratti in mice. J Helminthol 1995;69:119-123.
- 9. Korenaga M, Hitoshi Y, Yamaguchi N, Sato Y, Takatsu K, Tada I. The role of interleukin-5 in protective immunity to Strongyloides venezuelensis infection in mice. Immunology 1991;72:502-507.
- 10. Little MD. Comparative morphology of six species of Strongyloides (Nematoda) andredefinition of the genus. J Parasitol 1966;52:69-84.
- 11. Martin RJ. Modes of action of anthelmintic drugs. Vet J 1997;154:11-34.
- 12. Sato Y, Toma H. Strongyloides venezuelensis infections in mice. Int J Parasitol 1990;20:57-62.
- 13. Shi BB, Ishikawa N, Khan AI, Tsuchiya K, Horii Y, Nawa Y. Strongyloides venezuelensis infection in Syrian golden hamster, Mesocricetus auratus, with reference to the phenotype of intestinal mucosal mast cells. Parasite Immunol 1994;16:545-551.
- 14. Solomon MS, Haley AJ. Biology of the rat nematode Nippostrongylus brasiliensis (Travassos, 1914). Characteristics of N. brasiliensis after serial passage in the laboratory mouse. J Parasitol 1966;52:237-241.
- 15. Taira N, Hirooka M, Saeki H. Isolation of Strongyloides venezuelensis from Rattus norvegicus in Kagoshima Prefecture. J Vet Med Sci 1994;56:255-258.
- 16. Taira N, Nakamura Y, Almedia MA, Saeki H. Massive experimental infection with Strongyloides venezuelensis in rats and absence of sudden death. J Vet Med Sci 1995;57:855-858.
- 17. Tsuji N, Nakamura Y, Taira N. Long-lasting parasitism of Strongyloides venezuelensis in Mongolian gerbils (Meriones unguiculatus). J Parasitol 1993;79:305-307.
- 18. Wertheim G. Growth and development of Strongyloides venezuelensis Brumpt, 1934 in the albino rat. Parasitology 1970a;61:381-388.
- 19. Wertheim G. Experimental concurrent infections with Strongyloides ratti and S. venezuelensis in laboratory rats. Parasitology 1970b;61:389-395.
Fig. 1Recovery rate of migrating stage larvae (MSL3) from the lungs and adult worms from the small intestines of Fischer (F344) rats after infection with 1,000 L3 of Strongyloides venezuelensis harvested from nutrient broth culture (NBC), fecal culture (FC) and filter paper culture (FPC). Each point represents the percent ± SD of 5 rats.

Fig. 2Fecundity of parasitic females recovered from Fischer (F344) rats after infection with 1,000 L3 of Strongyloides venezuelensis obtained from nutrient broth culture (NBC), fecal culture (FC) and filter paper culture (FPC). Each point represents the mean ± SD of 50 worms. *P<0.05.

Fig. 3Kinetics of fecal egg output in Fischer (F344) rats after infection with 1,000 L3 of Strongyloides venezuelensis obtained from nutrient broth culture (NBC), fecal culture (FC) and filter paper culture (FPC). Each point represents the mean ± SD of 5 rats.
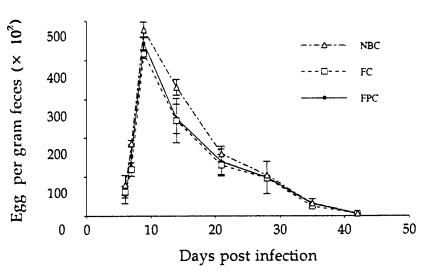
Table 1.Comparison of morphological dimensions among Strongyloides venezuelensis L3 stage obtained from three different in vitro culture methods
Table 1.
|
Parameters (in μm) |
In vitro culture methods
|
|
NBCa)
|
FCb)
|
FPCc)
|
|
Body length |
503.3 ± 31.0 |
509.9 ± 35.0 |
597.3 ± 32.2e)
|
|
Esophagus length |
234.6 ± 15.1 |
238.6 ± 14.3 |
266.6 ± 10.9 |
|
Tail length |
52.8 ± 3.1 |
55.6 ± 4.9 |
62.3 ± 3.2 |
|
Body width at E-I junctiond)
|
15.3 ± 1.3 |
15.8 ± 1.0 |
17.7 ± 0.7 |
Table 2.Comparison of morphological dimensions among Strongyloides venezuelensis adults recovered from Fischer (F344) rats on day 7 post-infection with 1,000 L3 obtained from three different culture methods
Table 2.
|
Parameters (in μm) |
Adult worms corresponding to the L3 obtained from
|
|
NBCa)
|
FCb)
|
FPCc)
|
|
Length of: |
|
|
|
|
Body |
2732.5 ± 169.9e)
|
2600.5 ± 172.4 |
2777.5 ± 204.4e)
|
|
Esophagus |
622.9 ± 49.2 |
594.4 ± 26.4 |
666.9 ± 42.0 |
|
Tail |
50.9 ± 3.0 |
47.4 ± 3.2 |
52.1 ± 3.3 |
|
Mouth to vulva |
1707.8 ± 74.9 |
1667.2 ± 77.4 |
1742.2 ± 106.3 |
|
Width at: |
|
|
|
|
E-I junctiond)
|
32.5 ± 1.7 |
31.1 ± 1.1 |
32.0 ± 2.3 |
|
Vulva |
41.9 ± 1.8 |
41.3 ± 1.2 |
41.4 ± 1.8 |
|
Anus |
20.1 ± 1.3 |
20.1 ± 1.3 |
19.1 ± 1.5 |
Table 3.Worm burdens in Fischer (F344) rats at weekly interval after subcutaneous infection with 1,000 L3 of Strongyloides venezuelensis harvested from three different in vitro culture methods
Table 3.
|
Day Post-infection |
Worm burdens corresponding to the culture methods
|
|
NBCa)
|
FCb)
|
FPCc)
|
|
7 |
166.6 ± 33.5 |
153.6 ± 46.6 |
173.8 ± 35.5 |
|
14 |
159.2 ± 75.5 |
151.4 ± 75.9 |
170.0 ± 76.1 |
|
21 |
112.0 ± 18.9 |
111.2 ± 22.8 |
132.0 ± 35.3 |
|
28 |
83.6 ± 24.7 |
79.2 ± 21.6 |
86.4 ± 24.2 |
|
35 |
32.0 ± 12.7 |
31.0 ± 12.7 |
39.2 ± 16.7 |
|
42 |
10.2 ± 2.6 |
9.8 ± 4.3 |
12.8 ± 4.4 |
|
49 |
0 |
0 |
0 |