Abstract
A total of nine Korean native kids and two Corriedale lambs, 1-20 days old, were each inoculated per os with a single dose of 2 × 107 oocysts of Cryptosporidium muris (strain MCR) originated from mice to elucidate the kinetics and developmental stages of the coccidium in small ruminants. Irrespective of host's age, the prepatent period for both animals ranged from 19 to 35 days (28.1 days, on the average) and the patent period 16-85 days (47.8 days), and the total oocyst outputs showed enormous differences. Infection with greater numbers of oocyst outputs was not ordinarily established by transmission experiments. Oocysts discharged from the kids retained their infectivity by the mouse titration method. The immunogenicity of the coccidium and oocyst reproduction were proven by challenge infection and administration of prednisolone acetate, respectively. All the developmental stages of the coccidium in parasitophorous vacuoles were found by transmission electron microscopy in the pits of the gastric glands of a kid inoculated with oocysts and then necropsied on day 44 postinoculation. It indicated the full course of the host-parasite relationship in kids and lambs as well as mice.
-
Key words: Cryptosporidium muris (strain MCR), kid, lamb, oocyst production, endogenous development
INTRODUCTION
The majority of cryptosporidiosis in man and animals have been documented as intestinal cryptosporidiosis by
C. parvum. Infection with
C. muris in cattle may be asymptomatic or associated with transient symptom, therefore one has no concern for relating to the impact of the parasite on it's hosts. Recently,
C. muris was rediscovered in the abomasum of cattle that had failed to eat well and failed to gain body weight optimally (
Anderson, 1987). Thereafter, Esteban and Anderson (
1995) reported that cows shedding
C. muris oocysts produced significantly less milk (approximately 3.2 kg/day) and that mean daily milk production was significantly associated with shedding status of oocysts. The protozoon resides in the peptic glands of the abomasum, causing dilation of the glands and mucosal hypertrophy. Microvilli of gland epithelial cells are destroyed at attachment sites of the parasite. Plasma pepsinogen concentrations are twice as high in parasitized than in non-parasitized animals (
Anderson, 1987 &
1989).
Even though cows shedding
C. muris oocysts produced significantly less milk with decreased growth rate, no reports, so far, have been published on the kinetics in details of
C. muris infection in ruminants excepting fragmentary delineations (
Anderson, 1987 &
1988). It is reasonable to use calf as an experimental animal to study the basic relationship of low productivity in cattle attributed to the protozoon. However, calf is not suitable to feeding and maintaining due to its large size in addition to low establishment (success rate) of transmission experiment (
Anderson, 1988). Therefore, kids and lambs were selected as an animal model instead of calf. This has been examined by assessing the life cycle comprising of oocyst production and entity of developmental stages of the parasite in the abomasum of ruminants throughout the transmission experiments. The immunogenicity of the coccidium and oocyst reproduction were observed to elucidate the relationship between abomasal cryptosporidiosis and the protozoon.
MATERIALS AND METHODS
Animals used for the transmission experiments were Korean native kids and Corriedale lambs delivered from their dams free from oocyst discharge. One- to twenty-day-old nine kids and two lambs were inoculated per os with a single dose of 2 × 10
7 oocysts of
C. muris (strain MCR) originated from mice, as previously described (
Rhee et al., 1995). Oocysts have been maintained in our laboratory since 1990. Experimental animals were separately housed in wire-floored cages with concentrates, roughage and fresh water available
ad libitum. The cages were placed on trays containing 5 mm depth of water to keep the feces wet.
To determine the infectivity of the oocysts discharged from the kids to mice. 10 SPF ICR mice, 3-week-old, were each inoculated orally with a single dose of 2 × 106 oocysts originated from the kids.
While, three days after oocyst shedding ceased, 2 kids that had shed small numbers of oocysts were intramuscularly injected with 55 mg prednisolone acetate daily for 14 days to reproduce oocysts. After complete shedding of oocysts, a lamb and a kid that had shed a large number of oocysts were each challenged orally with a single dose of 2 × 107 oocysts generated from the lamb or the kid to observe the immunogenicity of the parasite. To compare morphological dimension of oocysts in relation to their origin, 50 oocysts from each origin were measured by an ocular micrometer.
Following inoculations, the procedures used for the feeding of the mice and the examination of fecal samples of the ruminants and mice were subjected to those described previously by Rhee et al. (
1995).
To observe the developmental stages of the parasite, a 2-week-old kid inoculated orally with a single dose of 2 × 107 oocysts was necropsied on day 44 postinoculation (PI), when discharged 6 × 107 oocysts/day. The abomasal segments about 0.5 cm in each length were removed from peptic glandular region (spiral fold), fixed in 10% neutral formalin, and submitted to routine histologic processing. Paraffin-embedded sections were stained with hematoxylin and eosin, and PAS stain. In addition, the abomasal segments were fixed in Karnousky fixative (pH 7.4). The specimens were then washed in 0.1 M cacodylate buffer, postfixed in 2% sodium tetroxide solution in cacodylate buffer, and dehydrated in an ascending ethanol series. They were embedded in epoxy resin and sectioned with a diamond knife on a ultramicrotome. The ultrathin sections were stained with 20% uranyl acetate and examined with a JEM 1010 transmission electron microscope.
RESULTS
Discharge pattern of oocyst in feces
The pattern of oocyst shedding in
C. muris infection in 9 kids and 2 lambs is depicted in
Table 1. Each animal began to discharg oocysts on the 19th and 35th days (the 28.1th day, on the average) PI depending on the cases. The total oocyst outputs were different among the individual kids. The maximum oocyst counts (500-600 × 10
6) were recorded from three kids (Case Nos. 1, 2 and 11), while a medium numbers of oocysts (10-30 × 10
6) were counted from another three kids (Case Nos. 3, 4 and 5). However, the remaining three kids (Case Nos. 6, 7 and 8) discharged only a few oocysts in their fecal samples. The lambs also showed different oocyst outputs. As many as 304 × 10
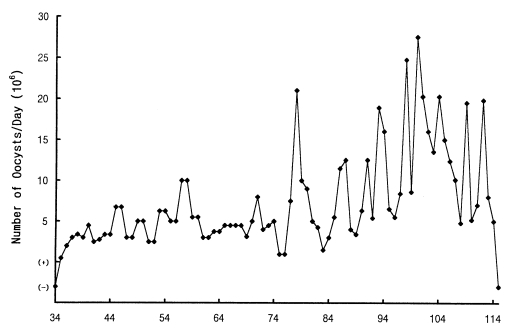
6 oocysts were recovered from one lamb (Case No. 9), while the other (Case No. 10) shed small numbers of oocysts. The patent periods for all animals ranged from 34 to 85 days, excepting a patency of 16 days in a lamb. It appears that the kids show longer patency than the lambs. In the first 2 cases of kids with greater numbers of oocyst shedding on comparable level, peak oocyst production with an undulating output occurred on the 78th and 112th days PI and declined rapidly thereafter until the oocysts were no longer present (
Fig. 1). In most cases of the remainder, oocysts were seldom detected about 60 days PI using a Fuchus-Rosenthal hemocytometer, however, qualitative determination by fecal notation and Kinyoun's modified acid-fast staining methods detected low levels of oocyst shedding up to about 70 days PI. The kids with a moderate oocyst output revealed some degree of undulating fashion of oocyst production over a period from 25th to 60th days PI.
All the mice inoculated with oocysts derived from kids excreted numerous oocysts (1 × 10
7/head/day in peak oocyst production period). In this occasion, the coccidium had the prepatent period of 9-11 days and the patent period of 60-69 days. Six days following the first prednisolone treatment, 2 kids (Case Nos. 6 and 8) that had shed small numbers of oocysts discharged a few uncountable oocysts by 7 days post-unmedication. In challenge infection, oocysts were not detected in the feces of a lamb (Case No. 9) and a kid (Case No. 3) each inoculated primarily with 2 × 10
7 oocysts at any time. As shown in
Table 2, morphological dimension of oocysts from mouse to goat model system was similar to that from goat to mouse model, whereas oocysts in both systems were somewhat smaller than those of strain RN 66 and MCR of
C. muris.
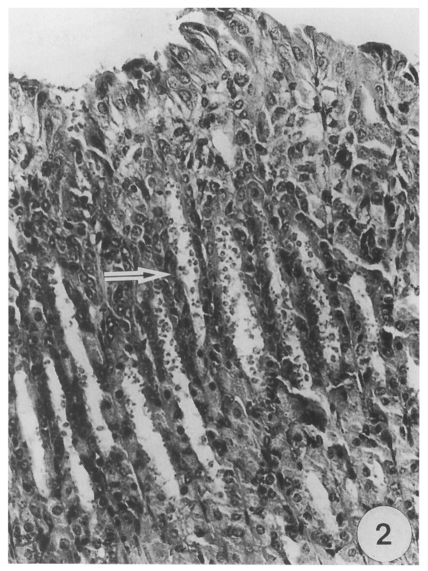
On light microscopy, Cryptosporidia were seen in the abomasal peptic glands of an infected kid. Most glands were found infected, and various developmental stages were observed from the apex to the orifice of the glands (
Fig. 2). Under transmission electron microscopy, all developmental stages in the parasitophorous vacuoles (PV) were found in the pits of the gastric glands. The endogenous development of the parasite occurred in the microvilli of the surface mucus cells of the gastric glands. A projection of the parasite, which was called a "knob like projection" by Tyzzer (
1910), was seen at the attachment site to the host cells. The outer aspect of the projection was surrounded by a thick, filamentous process of the host cell.
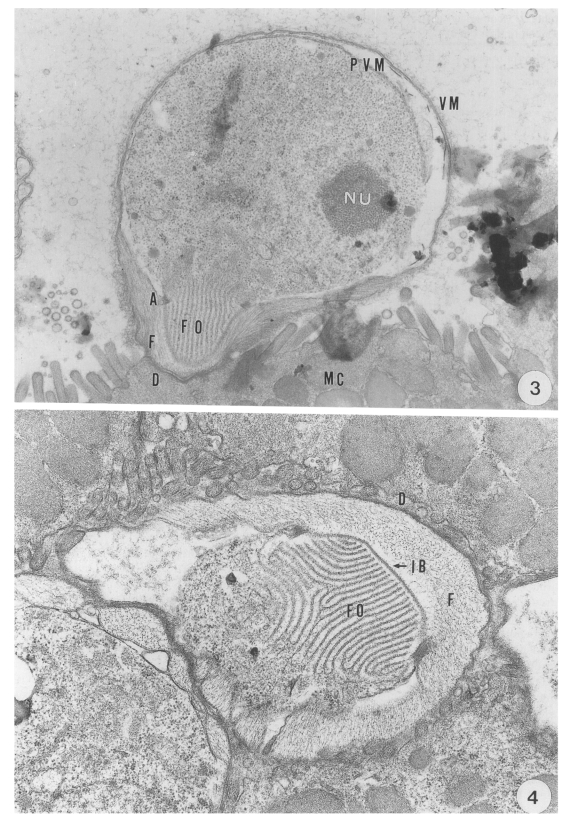
The round trophozoite; 5.1 (5.0-5.2) × 4.6 (4.4-4.8) µm in size, had a relatively large nucleus with a prominent nucleolus. The trophozoite, which was covered by a pellicle with a double membrane, was enclosed by the inner parasitophorous vacuolar membrane (PVM) and the outer villous membrane (VM) originating from the microvillus of the host cell. Membranous lamellae of a feeder organelle occupied the inner part of the projection of the trophozoite. The feeder organelle appeared to be in contact with the cytoplasmic membrane of the coccidium at an annular ring. The annular ring was seen at a junction consisting of the cytoplasmic membrane of the coccidium and the PVM- This junctional complex appeared to anchor the periphery of the feeder organelle firmly to the base of the PV (
Fig. 3). A dense band was seen at the base of the microvillous area where the parasite had invaded. An indented border separated the projection from the filamentous process of the host cell (
Fig. 4).
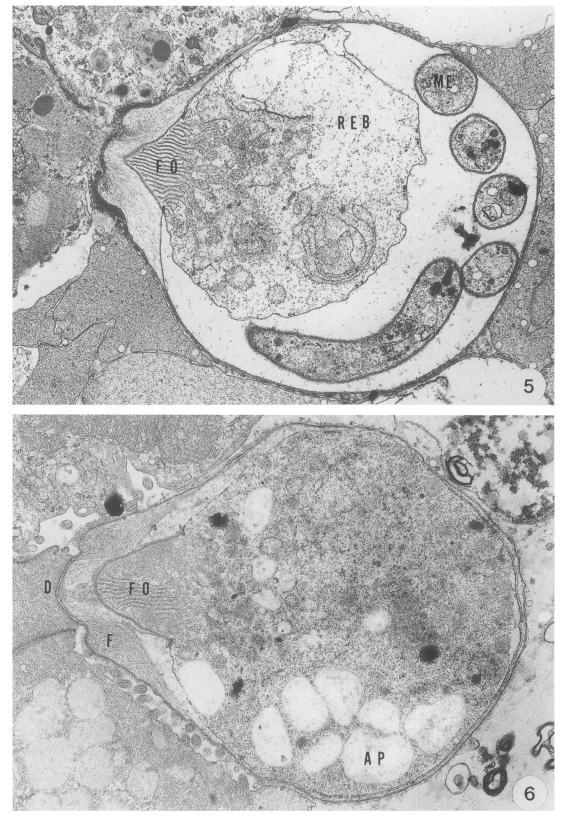
The trophozoite developed into the meront, in which eight merozoites were formed following three nuclear division. Various developmental stages of the meront measured 6.2 (5.4-6.8) × 5.4 (5.2-5.9) µm. The meront contained developing merozoites, a mass of residual body and a well developed feeder organelle in the PV (
Fig. 5). As merogony proceeded, the residual body of the meront was reduced. The fully developed merozoites, measuring 8.2 to 9.6 by 1.2 µm, in the PV were banana-shaped, and covered with a three unit membranous pellicle.
The macrogametes measured 7.1 (6.8-7.4) × 6.3 (5.9-6.8) µm, and the feeder organelle was well developed. As the parasite developed, amylopectin bodies were distributed throughout the cytoplasm, and wall-forming bodies containing material of different electron densities became more numerous and distributed in the peripheral cytoplasm. The parasite became round at the mature stage (
Fig. 6).
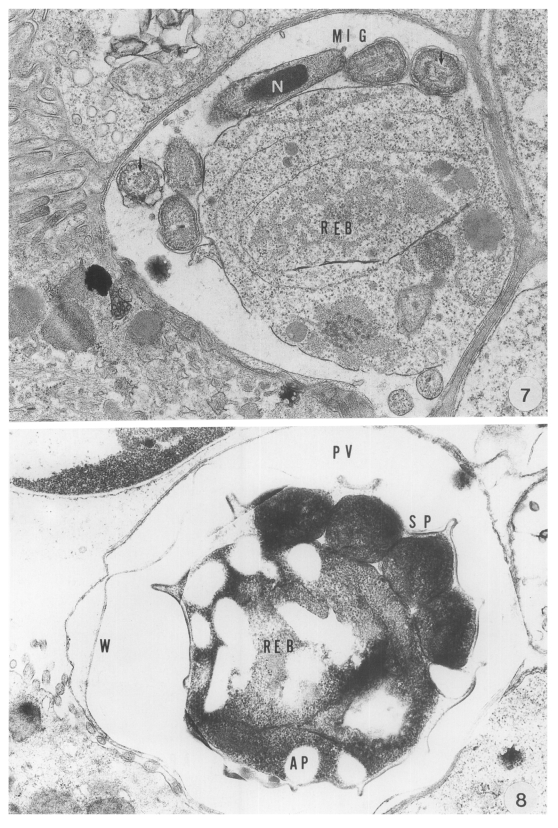
The microgametocyte was identified by the formation of microgametes and a large residual body. The microgametes were formed around the residual body. The parasites were produced by budding from the large microgametocyte: 16 microgametes were formed by microgametogenesis. The anterior part of the parasites was slightly expanded and a compact nucleus was surrounded by microtubules. Each parasite had a large nucleus and microtubules (arrows) paralleled to the long axis in its cytoplasm (
Fig. 7).
The oocyst, measuring 8.6 (7.8-9.0) × 6.5 (5.8-7.0) µm, was easily distinguished by a conspicuous oocyst wall composed of a double- or four-layered membranes. Sporogony took place in the PV, resulting in the formation of four sporozoites each of which had a nucleus, electron dense bodies and vacuoles in the cytoplasm. The mature sporozoite was banana-shaped, and its cross-section showed a round appearance (
Fig. 8).
DISCUSSION
Although the contention for taxonomy is continued until current, C. muris and C. parvum are valid, and the organism discovered in the abomasum of cattle is C. muris because ultrastructure of developmental stages, location of parasitism and the prepatent and patent periods differ from each other enormously.
Age-dependent resistance to
Cryptosporidium spp. or susceptibility to cryptosporidiosis of animals varies with the species of protozoa as well as. age of the host. Lambs became resistant to experimental infection with
C. parvum isolated from calves by about 15-20 days of age, and those infected at 30 days of age became infected with the organism and shed oocysts for a brief periods (1-2 days), but did not develop clinical signs of disease or growth retardation (
Tzipori et al,, 1981). Studies of
C. parvum infection in several strains of laboratory mice have demonstrated that 4- to 6-day-old mice were more susceptible to experimental infection than those of 21 or 42 days old (
Sherwood et al, 1982;
Heine et al., 1984). Sreter et al (
1995) indicated that both innate and acquired resistances to
C. baileyi were age-related in chickens.
On the other hand, we corroborated that susceptibilities to experimental infection with
C. muris (strain MCR) in mice at 3, 12 and 15 wk of age were similar (Rhee et al., unpublished observations).
Cryptosporidium muris appears to be associated with mild diarrhea in cattle of all ages, especially young animals (
Upton and Current, 1985). Anderson (
1988) described that abomasal cryptosporidiosis was produced in only one of 18 calves exposed 2 hr pre-clostrally to millions of fresh oocysts without presenting data in details. Given the data presented here, although it is well known that younger animals are more susceptible to
Cryptosporidium infection, susceptibility to
C. muris infection in kids and lambs may be irrespective of age like mice, because of a low relationship between age and intensity of oocyst outputs. However, we could not conclude age-independent resistance to
C. muris in kids and lambs because the number of experimental animals are not sufficient.
Infection with
C. muris appears to be chronic in cattle: the pattern of shedding oocysts in the steers was continuous over a period of 4 months and as of his writing, he had possession of three cattle which had shed
C. muris oocyst for over a year at the rate of about one million per gram of feces (
Anderson, 1987 &
1989). However, the prepatent and patent periods of the parasite for ruminants have not been established throughout transmission experiment as yet.
Our previous work (
Rhee et al., 1995) revealed that a patent period ranged from 61 to 64 days with a prepatent period of 9-11 days for mice inoculated orally with various doses of
C. muris (strain MCR) oocyst. While, Iseki et al. (
1989) intimated that the prepatent and patent periods of
C. muris (strain RN 66) for mice, guinea pigs, rabbits, dogs and cats were exceedingly different from each other. Based on the present findings, the prepatent and patent periods for kids and lambs were longer than those reported for various laboratory animals by Iseki et al. (
1989) and Rhee et al (
1995).
The significant differences in patency and the total oocyst outputs were noted depending on individual hosts. The standard deviations of the means of patency and the total oocyst outputs were high (47.8 ± 21.1 days and 156.6 × 10
6 ± 253.6 × 10
6), whereas the lengths of the prepatent period were similar to one another. Those of the prepatent period was relatively low (28.1 ± 5.8 days), while there was no correlation between the length of the patent period and the total oocyst output, indicating a significant individual difference in terms of the effectiveness of the acquired resistance to
C. muris (
Table 1). Therefore, even by same strain of inoculum, the prepatent and patent periods of
C. muris vary significantly with the species of host.
In the present study, no oocysts were excreted from a lamb and a kid by challenge infections at any time, which implies that the immunogenicity of the parasite is very strong like mice. Moreover, we ascertained the infectivity of the oocysts discharged from the kids to mice and reappearance of latent Cryptosporidia after primary infection by prednisolone acetate administration like mice. However, it is concluded that ruminants such as cattle, goat and sheep are not suitable hosts for the protozoon because infection with greater numbers of oocyst output was not found to occur with a familar findings in ruminants.
The present study primarily documented that the ultrastructural features of the attachment site of the parasite to the mucus cells differed remarkably from those of
C. parvum in the abomasum of ruminants. A unique structure of the anterior projection of the parasite was seen identical with that observed in the stomach of mice, as previously described by Uni et al. (
1987) and Rhee et al (
1991b). Moreover, the size of the parasite was greater at each developmental stage than that of
C. parvum. In addition, massive populations of this cryptosporidia were seen in the abomasal peptic glands, most of which were found infected with them.
Therefore, the present paper clarified the full course of the host-parasite interaction in ruminants throughout the transmission experiment and ultrastructure of the developmental stages with confirming greater numbers of cryptosporidia in the gastric peptic glands and fecal samples of kids and lambs.
ACKNOWLEDGEMENT
We are grateful to Dr. Bruce C. Anderson, Canine Veterinary Teaching Center, University of Idaho, USA, for offering his invaluable references regarding our study and photographs of preparation for abomasal cryptosporidiosis.
References
- 1. Anderson BC. Abomasal cryptosporidiosis in cattle. Vet Pathol 1987;24:235-238.
- 2. Anderson BC. Gastric cryptosporidiosis of feeder cattle, beef cows and dairy cows. Bovine Practioner 1988;23:99-101.
- 3. Anderson BC. In Angus KW, Blewett DA eds, Cryptosporidium spp. in cattle. Cryptosporidiosis. 1989. Proc First Intl Workshop. Edinburgh, Scotland, UK: 55-63.
- 4. Anderson BC. A preliminary report on prevalence of Cryptosporidium muris oocysts in dairy cattle feces. California Veterinarian 1990;44(1):11-12.
- 5. Anderson BC ed, Bovine gastric and intestinal cryptosporidiosis: Present situation. 1992. Proc 24th Ann Convention Am Ass Bovine Practitioners. Florida. USA: l5-18.
- 6. Esteban E, Anderson BC. Cryptosporidium muris: Prevalence, persistency, and detrimental effect on milk production in a drylot dairy. J Dairy Sci 1995;78:1068-1072.
- 7. Heine J, Moon HW, Woodmansee DB. Persistent Cryptosporidium infection in congenitally athymic (nude) mice. Infect Immun 1984;43:856-859.
- 8. Iseki M, Maekawa T, Moriya K, Uni S, Takada S. Infectivity of Cryptosporidium muris (strain RN 66) in various laboratory animals. Parasitol Res 1989;75:218-222.
- 9. Nakai Y, Kaeta Y, Takesima S, Ando T. Isolation of Cryptosporidium oocysts from adult cattle and their experimental infection to mice and rats. Jpn J Parasitol 1996;45(suppl):126.
- 10. Rhee JK, Seu YS, Park BK. Isolation and identification of Cryptosporidium from various animals in Korea I. Prevalence of Cryptosporidium in various animals. Korean J Parasitol 1991a;29(2):139-148.
- 11. Rhee JK, Seu YS, Park BK. Isolation and identification of Cryptosporidium from various animals in Korea II. Identification of Cryptosporidium muris from mice. Korean J Parasitol 1991b;29(2):149-159.
- 12. Rhee JK, Yook SY, Park BK. Oocyst production and immunogenicity of Cryptosporidium muris (strain MCR) in mice. Korean J Parasitol 1995;33(4):377-382.
- 13. Sherwood D, Angus KW, Snodgrass DR, Tzipori S. Experimental cryptosporidiosis in laboratory mice. Infect Immun 1982;38:471-475.
- 14. Sreter T, Varga I, Bekesi L. Age-dependent resistance to Cryptosporidium baileyi. J Parasitol 1995;81(5):827-829.
- 15. Tyzzer EE. An extracellular coccidium, Cryptosporidium muris (gen. et sp. nov.), of the gastric glands of the common mouse. J Med Res 1910;23:487-509.
- 16. Tzipori S, Angus KW, Gray EW, Campbell I, Allan F. Diarrhea in lambs experimentally infected with Cryptosporidium isolated from calves. Am J Vet Res 1981;42:1400-1404.
- 17. Uni S, Iseki M, Maekawa T, Moriya K, Takada S. Ultrastructure of Cryptosporidium muris (strain RN 66) parasitizing the murine stomach. Parasitol Res 1987;74:123-132.
- 18. Upton SJ, Current WL. The species of Cryptosporidium (Apicomplexa: Cryptosporidiidae) infecting mammals. J Parasitol 1985;71(5):625-629.
Fig. 1Mean daily oocyst excretion of two kids each inoculated orally with 2 × 107 oocysts of C. muris (strain MCR) at 20 days of age. (+) and (-) indicate that less than 103 oocysts were detected and oocysts were not detected, respectively.

Fig. 2Most abomasal peptic glands (arrow) of an infected kid are filled with large numbers of various Cryptosporidial forms, × 350, HE.

Fig. 3-8
Transmission electron micrographs showing the endogenous development of C. muris (strain MCR) parasitizing the abomasum of a kid. Fig. 3. A trophozoite growing in the microvilli of the surface mucus cells of the abomasal peptic glands, × 10,000. Fig. 4. An indented border separating the parasite projection consisting of feeder organelle from the filamentous process of the host cells, × 12,000.
Abbreviations: A, annular ring; AP, amylopectin body; D, dense band; F, filamentous process: FO, feeder organelle; IB, indented border; MC, mucus cell; ME, merozoite; MIG, microgamete: N, nucleus; NU, nucleolus: REB, residual body: PV, parasitophorous vacuole; PVM, parasitophorous vacuolar membrane; SP, sporozoite; VM, villous membrane; W, oocyst wall; WB, wall-forming body
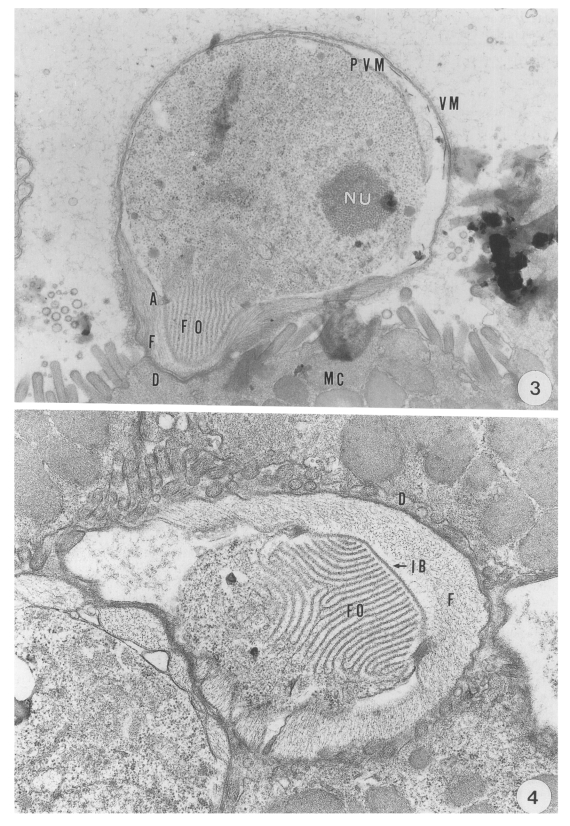
Fig. 3-8
Transmission electron micrographs showing the endogenous development of C. muris (strain MCR) parasitizing the abomasum of a kid. Fig. 5. A younger meront growing in the microvilli, showing a banana-shaped and four round cross-sections of merozoites, × 6,000. Fig. 6. A macrogamete showing numerous amylopectin bodies in the microvilli, × 8,000.
Abbreviations: A, annular ring; AP, amylopectin body; D, dense band; F, filamentous process: FO, feeder organelle; IB, indented border; MC, mucus cell; ME, merozoite; MIG, microgamete: N, nucleus; NU, nucleolus: REB, residual body: PV, parasitophorous vacuole; PVM, parasitophorous vacuolar membrane; SP, sporozoite; VM, villous membrane; W, oocyst wall; WB, wall-forming body

Fig. 3-8
Transmission electron micrographs showing the endogenous development of C. muris (strain MCR) parasitizing the abomasum of a kid. Fig. 7. A microgametocyte comprising eight microgametes in the microvilli, × 10,000. Fig. 8. An oocyst at the late stage in the microvilli, showing large parasitophorous vacuole, × 6,000.
Abbreviations: A, annular ring; AP, amylopectin body; D, dense band; F, filamentous process: FO, feeder organelle; IB, indented border; MC, mucus cell; ME, merozoite; MIG, microgamete: N, nucleus; NU, nucleolus: REB, residual body: PV, parasitophorous vacuole; PVM, parasitophorous vacuolar membrane; SP, sporozoite; VM, villous membrane; W, oocyst wall; WB, wall-forming body
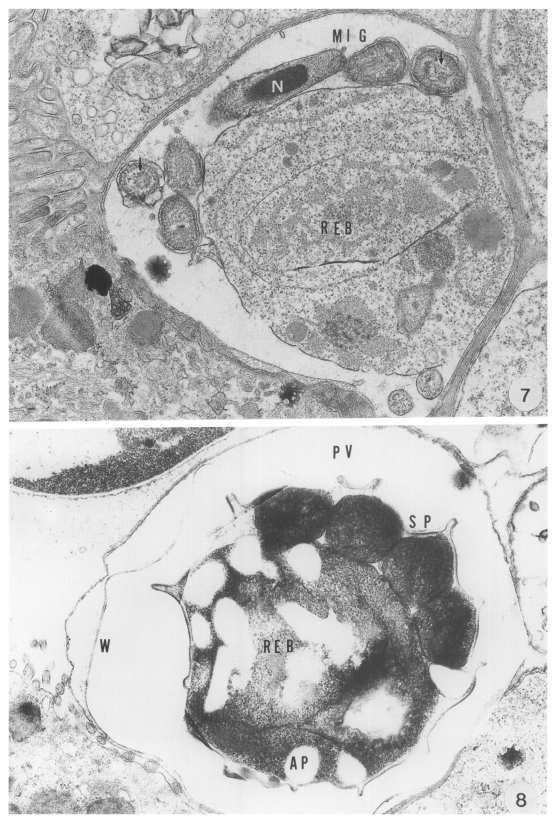
Table 1.The length of the prepatent and patent periods and the total oocyst excretion of ruminants inoculated with 2 × 107 Cryptosporidium muris (strain MCR) oocysts
Table 1.
|
Case No. |
Host |
Age (days) |
Prepalent period (days) |
Patent period (days) |
Total oocyst output (× 106) |
Remarks |
|
1 |
goata)
|
20 |
32 |
83 |
594.8 |
|
|
2 |
goat |
20 |
30 |
85 |
613.5 |
|
|
3 |
goat |
14 |
24 |
45 |
29.9 |
challenge |
|
4 |
goat |
14 |
26 |
43 |
13.7 |
|
|
5 |
goat |
7 |
19 |
34 |
9.7 |
|
|
6 |
goal |
3 |
34 |
45 |
small numbersd)
|
reappearance |
|
7 |
goat |
3 |
27 |
44 |
small numbers |
|
|
8 |
goat |
3 |
35 |
44 |
small numbers |
reappearance |
|
9 |
sheepb)
|
1 |
28 |
38 |
304.3 |
challenge |
|
10 |
sheep |
1 |
35 |
16 |
small numbers |
|
|
11e)
|
goat |
15 |
19 |
> 25 |
> 564.5 |
victimization |
|
|
mean |
|
9.2 |
28.1 |
47.8 |
156.6 |
|
|
± SD |
|
7.5 |
5.8 |
21.1 |
253.6 |
|
|
CV (%)c)
|
|
82.0 |
20.6 |
44.1 |
161.9 |
|
Table 2.Comparison of dimension of oocysts in relation to their origin
Table 2.
|
Strains or origins |
Length (μm) |
Width (μm) |
|
RN 66 |
8.42 ± 0.41 |
6.54 ± 0.37 |
|
MCR |
7.80 ± 0.56 |
5.89 ± 0.36 |
|
Mouse/goat system |
7.70 + 0,18 |
5.22 ± 0.19 |
|
Goat/mouse system |
7.63 ± 0.22 |
5.20 ± 0.14 |