AbstractStandard- and large-sized eggs of Trichuris trichiura were found in the feces of schoolchildren in Yangon, Myanmar during epidemiological surveys and mass deworming with albendazole in 2017–2019. The standard-sized eggs were identified as those of T. trichiura, but it was necessary to exclude the possibility of the large-sized eggs belonging to Trichuris vulpis, a dog whipworm. We conducted morphological and molecular studies to determine the species of the 2 types of Trichuris eggs. Individual eggs of both sizes were isolated from Kato-Katz fecal smears (n=20) and mechanically destroyed using a 23G injection needle. Nuclear DNA was extracted, and the 18S rRNA region was sequenced in 15 standard-sized eggs and 15 large-sized eggs. The average size of standard-sized eggs (T. trichiura) was 55.2×26.1 μm (range: 51.7–57.6×21.3–28.0 μm; n=97), whereas the size of large-sized eggs was 69.3×32.0 μm (range: 65.1–76.4×30.1–34.5 μm; n=20), slightly smaller than the known size of T. vulpis. Regarding standard-sized eggs, the 18S rRNA nucleotide sequences exhibited 100% homology with T. trichiura deposited in GenBank and 88.6–90.5% homology with T. vulpis. Regarding large-sized eggs, the nucleotide sequences showed 99.8–100% homology with T. trichiura in GenBank and 89.6–90.7% homology with T. vulpis. Both standard- and large-sized eggs of Trichuris spp. found in Myanmar schoolchildren during 2017–2019 were morphologically and molecularly confirmed to belong to T. trichiura. The conversion of eggs from smaller to large sizes might be due to anthelmintic treatments with albendazole.
Trichuris trichiura, the human whipworm, is found in tropical and subtropical regions with poor public health and living conditions, and it is estimated to infect approximately 600–800 million people globally [1,2]. Although many infected individuals do not show symptoms, severe infections can lead to chronic mucous diarrhea, malnutrition, growth retardation, iron deficiency anemia (caused by intestinal bleeding), and even rectal prolapse [3,4]. The World Health Organization (WHO) classifies trichuriasis as a neglected tropical disease (NTD) [1,2].
In addition to human-specific whipworm, 20 Trichuris species infect animals, including Trichuris vulpis, the dog whipworm [5]. Human infections with T. vulpis have been rarely reported [5–9]. Diagnosis of these T. vulpis infections has been based on the identification of eggs markedly larger (70–99×30–47 μm) than those of T. trichiura (50–55×20–25 μm) [5–8]. Notably, treatment with anthelmintics, such as mebendazole, can also alter the size and shape of T. trichiura eggs found in the patient’s feces [10,11]. The appearance of large-sized Trichuris eggs has also been observed during albendazole and tribendimidine treatment [12], although some cases were suspected to be T. vulpis infections. Therefore, relying solely on egg size for diagnosing Trichuris spp. infection has been inconsistent and requires careful consideration [11].
Molecular studies have been conducted to distinguish between T. trichiura and T. vulpis using the sequence of small subunit ribosomal DNA (SSU rDNA) and internal transcribed spacer 1 (ITS-1) genes in Thailand [8] as well as PCR-restriction fragment length polymorphism (PCR-RFLP) patterns in Brazil, Cambodia, Cameroon, Ethiopia, Tanzania, and Vietnam [10]. In Thailand, out of 56 schoolchildren who tested positive for T. trichiura, 6 showed potential coinfection with T. vulpis [7]. Conversely, in Brazil, Cambodia, Ethiopia, Tanzania, and Vietnam, all 87 schoolchildren examined using molecular analysis were positive for T. trichiura alone [9]. In Cameroon, 23 and 7 children were analyzed using molecular techniques and found to be positive for T. trichiura and T. vulpis, respectively [9]. In addition, the internal transcribed spacer 2 (ITS-2), 18S ribosomal RNA (18S rRNA), and mitochondrial cytochrome c oxidase subunit 1 (cox1) have been used to differentiate T. trichiura from other Trichuridae nematodes [13]. Therefore, molecular studies are crucial for accurately diagnosing large-sized Trichuris eggs.
We conducted the “Korea-Myanmar Health Promotion Project for Elementary Schoolchildren of the Vulnerable Areas around Yangon, Myanmar” as an international parasite control initiative for 6 years (2014–2019), with mass drug administration (MDA) of albendazole (400 mg in a single dose). The control of Ascaris lumbricoides was successful during 2014–2016 [14]. However, trichuriasis control proved challenging even with 2–4 rounds of MDA per year during 2017–2019 [15]. Throughout this period, we observed the presence of large-sized Trichuris eggs alongside the standard-sized eggs of T. trichiura in the feces of a substantial number (>300) of schoolchildren. These eggs were comparable in size to those of T. vulpis. Therefore, it was necessary to exclude the possibility of mixed infections with both T. trichiura and T. vulpis in these schoolchildren.
The objective of the present study, conducted in 2017–2019, was to analyze the morphology and molecular characteristics of standard- and large-sized eggs of Trichuris spp. found in Kato-Katz (K-K) fecal smears from schoolchildren in the Yangon Region, Myanmar. Both the project and the K-K fecal examinations of the schoolchildren were approved by the Ethics Review Committee, Department of Medical Research, Ministry of Health and Sports, Yangon, Myanmar (ERC no.: 005117).
The surveyed areas consisted of 3 suburban districts (Shwe Pyi Thar, Twantay, and Kyauktan) of Yangon, Myanmar. To assess the prevalence of soil-transmitted helminths, we conducted K-K fecal examinations followed by MDA using albendazole targeting 1,724 schoolchildren (6–9 years old; gender ratio M:F=49:51) in June 2017. Follow-up examinations with repeated MDA were conducted 5 times in total (every 5–7 months) until November 2019. The overall outcomes of this control activity were reported previously [15]. For further study on the species of Trichuris eggs through morphological and molecular analyses, some of the K-K smears that tested positive for standard- and large-sized Trichuris spp. eggs were transported to the laboratory of the MediCheck Research Institute, Korea Association of Health Promotion, Seoul, Republic of Korea.
In total, 20 K-K smear slides from schoolchildren, containing both standard- and large-sized Trichuris eggs, were morphologically analyzed using an Olympus CKX41 microscope (Tokyo, Japan) equipped with a DP72 digital camera (Tokyo, Japan). Standard-sized eggs were defined as 50–60 μm in length and 20–30 μm in width, whereas large-sized eggs were defined as 65–80 μm in length and 25–35 μm in width. Length and width measurements were taken on the K-K smears using the CellSens standard v1.5 image analysis software on a Leica research microscope (Wetzlar, Germany) at 400×magnification.
After size measurement, the standard- and large-sized Trichuris eggs were isolated from the K-K smear slides. The cellophane paper attached to the slide was removed, and a Trichuris egg (standard- or large-sized) with surrounding fecal material was extracted and transferred to a Petri dish containing 0.85% saline solution. Each egg was individually washed with saline and carefully transferred onto the lid of a 1.5 ml sample tube. On the tube lid, the transferred egg was observed under a stereomicroscope and mechanically destroyed using a 23G injection needle to release the parasite germ cell substance.
The broken eggs were further processed for analysis of the nuclear genes, specifically the 18S rRNA. Genomic DNA was isolated using a DNeasy Blood & Tissue Kit (Qiagen, Hilden, Germany) following the spin-column protocol. The 18S rRNA gene was PCR-amplified on a C1000 Touch Thermal Cycler using the forward primer 965_F (5′-GGCGAT CAGATACCGCCCTAGTT-3′) and reverse primer 1573R_R (5′-TACAAAGGGCAG GGACGTAAT-3′) [13]. The thermal cycling profiles included an initial denaturation at 95°C for 15 min, followed by 40 cycles of denaturation at 95°C for 30 sec, annealing at 53°C for 30 sec, and primer extension at 72°C for 1 min, with a final extension at 72°C for 10 min [13]. The genomic DNA was isolated using 1.5% agarose gel electrophoresis. Sequencing of the 18S rRNA region was conducted at Macrogen (Seoul, Korea). The obtained sequences were compared with the GenBank reference sequences of T. trichiura and T. vulpis using the BLAST (http://blast.ncbi.nlm.nih.gov/Blast.cgi) with the Geneious ver. 11.1.5 (Biometers Ltd., Auckland, New Zealand).
Prior to the albendazole MDA, large-sized eggs of T. trichiura were not observed among the schoolchildren. However, after the MDA, both standard-sized (typical) eggs of T. trichiura and large-sized (atypical) Trichuris eggs were found in K-K slide samples (Figs. 1, 2). The standard-sized T. trichiura eggs had a barrel-shaped morphology with a thick eggshell, a single blastomere, and polar mucoid plugs at both ends (Fig. 1A–D). The large-sized eggs exhibited similar morphology to the standard-sized eggs but with larger dimensions. The larger eggs were often found in the same microscopic fields as the small typical T. trichiura eggs (Fig. 1B–D) or alongside other helminth eggs, such as fertilized (Fig. 1D) or unfertilized eggs of A. lumbricoides. The average size of the typical eggs was 55.2×26.1 μm (range: 51.7–57.6×21.3–28.0 μm; n=97) (box A in Fig. 2), whereas the large-sized eggs measured 69.3×32.0 μm (range: 65.1–76.4×30.1–34.5 μm; n=20) (box B in Fig. 2). The size ranges of the large-sized eggs in this study were slightly smaller than those of T. vulpis, although some overlaps in length and width were observed (box C in Fig. 2) [5–8].
The amplification of 18S rRNA genes from standard- and large-sized eggs yielded partial sequences of 368–437 bp and 426–443 bp, respectively. The sequences of 15 small-sized eggs (GenBank accession no.: OQ152555-OQ152569) clustered together with high homology (100%) and strong bootstrap support (Table 1; Supplementary Table S1). The pairwise percent identity between standard-sized eggs and T. trichiura in GenBank (GQ352548; Thailand) was high (100%), whereas the identity between standard-sized eggs and T. vulpis (GQ352556; Thailand) was only 88.6–90.5% (Table 1; Supplementary Table S1). Similarly, the sequences of 15 large-sized eggs (GenBank accession no.: OQ152579-OQ152593) formed a closely clustered group (almost 100% homologous) with high bootstrap values (Table 1; Supplementary Table S2). The large-sized eggs exhibited high homologies of 99.8–100% with T. trichiura in GenBank (GQ352548; Thailand), whereas the identity between large-sized eggs and T. vulpis (GQ352556; Thailand) was 89.6–90.7% (Table 1; Supplementary Table S2). Therefore, both the standard- and large-sized eggs of Trichuris spp. found in schoolchildren around the Yangon Region, Myanmar, were molecularly confirmed as T. trichiura eggs.
In this study, both the standard- and large-sized eggs of Trichuris found in the feces of schoolchildren around the Yangon Region, Myanmar were morphologically and molecularly identified as T. trichiura. The altered egg size is likely attributed to repeated sublethal dose treatments with albendazole during the control project. However, the presence of T. vulpis eggs mixed with T. trichiura eggs in a few schoolchildren (among others excluding 20 analyzed cases) cannot be completely ruled out and requires further confirmation.
The first report of large-sized eggs of Trichuris spp. in human stool was by Hall and Sonnenberg in 1956 [5]. They morphologically identified an adult whipworm as a female T. vulpis following treatment with gentian violet. Since then, several suspected cases of human T. vulpis infection have been documented [6–9,12,16]. However, most of these reports were based solely on egg sizes, >70 μm in length, and several researchers have questioned the true nature of these infections, suggesting that some of the large-sized eggs may actually be atypical eggs of T. trichiura [16]. Notably, the occurrence of large-sized eggs can be a normal phenomenon for T. trichiura (in approximately 20% of females), with these eggs being slightly smaller than those of T. vulpis [16]. In our study, the majority of large-sized eggs were smaller than those reported for T. vulpis in the literature [5–8], indicating atypical eggs of T. trichiura. Moreover, this was confirmed through molecular analysis of 18S rRNA sequences.
The size and morphology of T. trichiura eggs can also be altered, becoming enlarged and atypical, after treatment with anthelmintics, including mebendazole and albendazole, with such changes also occurring due to aging of the worms [10,11]. Wagner and Peña Chavarria [10] observed morphologically altered T. trichiura eggs at days 1–7 following mebendazole treatment. These alterations included changes in size, shape (e.g., losing the typical barrel form and disappearance of mucoid plugs), and discoloration of the egg yolk [10,17]. In addition, a high percentage of eggs in affected T. trichiura worms did not develop into larvae, indicating the ovicidal activity of mebendazole [17]. In an experimental human T. trichiura infection, the production of large-sized eggs was observed from 23-week after a single dose of mebendazole treatment and continued until 138-week (2.6 years) post-treatment [11]. Furthermore, the proportion of abnormal eggs increased with the number of sublethal repeated doses of mebendazole [11].
Similar increases in egg size and loss of developmental capacity of eggs have been observed in other nematode species after treatment with benzimidazole anthelmintics [18,19]. For instance, pigeons infected with the trichurid nematode Capillaria obsignata and treated with cambendazole excreted eggs that included abnormally larger eggs from day 21 post-infection [18]. In mice infected with Trichuris muris and sheep infected with Haemonchus contortus, eggs did not develop into larvae after treatment with febantel, a probenzimidazole agent, indicating its ovicidal activity [19].
Benzimidazole anthelmintics inhibit glucose metabolism, including glucose uptake and transport, which results in the starvation of parasites [20]. The drugs bind to intracellular tubulin and selectively inhibit microtubule-dependent transport functions in parasites [20]. Consequently, glycogen and ATP depletions occur in parasite cells, which are vital for their survival and reproduction. The drugs appear to act readily on the ovaries of parasites, as observed in C. obsignata [18]. However, both normal and abnormal (including large-sized) eggs were produced after anthelmintic treatment in C. obsignata [18] and T. trichiura [11]. It is speculated that sublethal damage in specific parts of the ovaries in T. trichiura affects only the eggs produced in these sites, leading to the production of large-sized eggs, whereas unaffected areas give rise to normal eggs [11]. Large-sized eggs of T. trichiura also exhibited a low embryonation rate, which may be linked to sublethal damage or malformation of the worm’s reproductive system [11]. However, further investigation is needed to understand the detailed mechanisms underlying the production of large-sized eggs in trichurid nematodes following anthelmintic treatment.
Possible correlations between the production of large-sized eggs by T. trichiura and the development of drug resistance in this whipworm have been suggested. Specifically, a single nucleotide polymorphism at codon 200 (possibly also at codons 167 and 198) of the β-tubulin gene has been implicated in benzimidazole anthelmintic resistance in T. trichiura [20]. However, no documented trials have examined the relationship between the mutation at the β-tubulin gene and the production of abnormal and deformed eggs, including large-sized eggs. Therefore, genetic studies on the β-tubulin gene in standard- and large-sized eggs of T. trichiura are warranted.
In our study, both standard- and large-sized eggs were detected in K-K fecal smears and were subsequently isolated from the smear slides. Conversely, Steinmann et al. [12] identified large-sized Trichuris eggs using a flotation technique, called FLOTAC but did not find them in K-K preparations. In contrast, Nejsum et al. [11] used the K-K technique to detect standard- and large-sized eggs of T. trichiura. Therefore, our study results align with those of Nejsum et al. [11].
Based on previous reports and the present study, several possibilities should be considered when observing Trichuris spp. eggs in fecal samples. These include changes in egg size due to anthelmintic drug treatment and/or worm aging, as well as infection with zoonotic whipworm species, including T. vulpis and others. To achieve an accurate diagnosis, it is necessary to employ various methods, including egg measurements, molecular analysis, and if possible, recovery of adult worms from the patient.
In conclusion, standard- and large-sized Trichuris eggs were detected in the feces of schoolchildren during a parasite control project that involved MDA of albendazole in the Yangon Region, Myanmar. To confirm that the large-sized eggs were not those of T. vulpis, we conducted morphological and molecular analyses on both types of eggs. The findings conclusively identified both types as T. trichiura and ruled out the presence of T. vulpis eggs. Moreover, it is highly likely that some T. trichiura eggs became larger as a result of albendazole anthelmintic treatments.
AcknowledgmentsWe appreciate the staff of the National Health Laboratory, Ministry of Health and Sports, Yangon, Myanmar who helped collection of fecal specimens from residents around the Yangon Region and the preparation of Kato-Katz smears during 2017–2019. Special thanks are given to all participants in this epidemiological study, particularly the schoolchildren. We also appreciate the staff of the Korea Association of Health Promotion (KAHP), Seoul, South Korea who helped with this project.
NotesAuthor contributions
Conceptualization: Jung BK, Chai JY
Data curation: Ryoo S, Jung BK, Hong S, Shin H, Song H, Sohn WM, Hong SJ, Chai JY
Formal analysis: Jung BK, Sohn WM, Hong SJ, Chai JY
Investigation: Ryoo S, Jung BK, Hong S, Shin H, Song H, Sohn WM, Hong SJ, Chai JY
Methodology: Ryoo S, Jung BK
Project administration: Kim HS, Ryu JY, Htoon TT, Tin TT, Chai JY
Resources: Kim HS, Ryu JY, Htoon TT, Tin TT
Software: Ryoo S, Jung BK
Supervision: Sohn WM, Hong SJ, Htoon TT, Tin TT, Chai JY
Validation: Jung BK, Chai JY
Visualization: Ryoo S, Jung BK, Chai JY
Writing-original draft: Ryoo S, Jung BK
Writing-Reviews & editing: Jung BK, Chai JY
Conflict of interestThe authors declare that there are no conflicts of interest related to this study.
References1. Hotez PJ, Fenwick A, Savioli L, Molyneux DH. Rescuing the bottom billion through control of neglected tropical diseases. Lancet 2009;373(9674):1570-1575
https://doi.org/10.1016/S0140-6736(09)60233-6
2. Dunn JC, Turner HC, Tun A, Anderson RM. Epidemiological surveys of, and research on, soil-transmitted helminths in Southeast Asia: a systematic review. Parasit Vectors 2016;9:31
https://doi.org/10.1186/s13071-016-1310-2
3. Ok KS, Kim YS, Song JH, Lee JH, Ryu SH, et al.
Trichuris trichiura infection diagnosed by colonoscopy: case reports and review of literature. Korean J Parasitol 2009;47(3):275-280
https://doi.org/10.3347/kjp.2009.47.3.275
4. Ramdath DD, Simeon DT, Wong MS, Grantham-McGregor SM. Iron status of schoolchildren with varying intensities of Trichuris trichiura infection. Parasitology 1995;110(3):347-351
https://doi.org/10.1017/s0031182000080938
5. Hall JE, Sonnenberg B. An apparent case of human infection with the whipworm of dogs, Trichuris vulpis (Froelich, 1789). J Parasitol 1956;42(2):197-199.
6. Dunn JJ, Columbus ST, Aldeen WE, Davis M, Carroll KC.
Trichuris vulpis recovered from a patient with chronic diarrhea and five dogs. J Clin Microbiol 2002;40(7):2703-2704
https://doi.org/10.1128/JCM.40.7.2703-2704.2002
7. Areekul P, Putaporntip C, Pattanawong U, Sitthicharoenchai P, Jongwutiwes S.
Trichuris vulpis and T. trichiura infections among schoolchildren of a rural community in northwestern Thailand: the possible role of dogs in disease transmission. Asian Biomed 2010;4(1):49-60
https://doi.org/10.2478/abm-2010-0006
8. Márquez-Navarro A, García-Bracamontes G, Álvarez-Fernández BE, Ávila-Caballero LP, Santos-Aranda I, et al.
Trichuris vulpis (Froelich, 1789) infection in a child: a case report. Korean J Parasitol 2012;50(1):69-71
https://doi.org/10.3347/kjp.2012.50.1.69
9. George S, Geldhof P, Albonico M, Ame SM, Bethony JM, et al. The molecular speciation of soil-transmitted helminth eggs collected from school children across six endemic countries. Trans R Soc Trop Med Hyg 2016;110(11):657-663
https://doi.org/10.1093/trstmh/trw078
10. Wangner ED, Peña Chavarria AP. Morphologically altered eggs of Trichuris trichiura following treatment with mebendazole. Am J Trop Med Hyg 1974;23(2):154-157
https://doi.org/10.4269/ajtmh.1974.23.154
11. Nejsum P, Andersen KL, Andersen SD, Thamsborg SM, Tejedor AM. Mebendazole treatment persistently alters the size profile and morphology of Trichuris trichiura eggs. Acta Trop 2020;204:105347
https://doi.org/10.1016/j.actatropica.2020.105347
12. Steinmann P, Rinaldi L, Cringoli G, Du ZW, Marti H, et al. Morphological diversity of Trichuris spp. eggs observed during an anthelmintic drug trial in Yunnan, China, and relative performance of parasitologic diagnostic tools. Acta Trop 2015;141(B):184-189
https://doi.org/10.1016/j.actatropica.2014.08.018
13. Guardone L, Deplazes P, Macchioni F, Magi M, Mathis A. Ribosomal and mitochondrial DNA analysis of Trichuridae nematodes of carnivores and small mammals. Vet Parasitol 2013;197(1–2):364-369
https://doi.org/10.1016/j.vetpar.2013.06.022
14. Chai JY, Sohn WM, Hong SJ, Jung BK, Hong S, et al. Effect of mass drug administration with a single dose of albendazole on Ascaris lumbricoides and Trichuris trichiura infection among schoolchildren in Yangon Region, Myanmar. Korean J Parasitol 2020;58(2):195-200
https://doi.org/10.3347/kjp.2020.58.2.195
15. Hong S, Jung BK, Song H, Cho J, Sohn WM, et al. Failure of repeated MDA with albendazole for trichuriasis control in schoolchildren of the Yangon Region, Myanmar. Korean J Parasitol 2021;59(6):607-613
https://doi.org/10.3347/kjp.2021.59.6.607
16. Yoshikawa H, Yamada M, Matsumoto Y, Yoshida Y. Variations in egg size of Trichuris trichiura
. Parasitol Res 1989;75(8):649-654
https://doi.org/10.1007/BF00930964
17. Wangner ED, Peña Chavarria AP. In vivo effects of a new anthelmintic, mebendazole (R-17, 635) on the eggs of Trichuris trichiura and hookworm. Am J Trop Med Hyg 1974;23(2):151-153.
18. Dorny P, Berghen P, Vercruysse J. Morphological changes in Capillaria obsignata (Nematoda: Trichuridae) eggs after treatment with cambendazole. Parasitol Res 1987;74(2):196-197
https://doi.org/10.1007/BF00536034
19. Thomas H. Ovacidal activity of febantel. N Z Vet J 1979;27(12):273-275
https://doi.org/10.1080/00480169.1979.34670
20. Chai JY, Jung BK, Hong SJ. Albendazole and mebendazole as anti-parasitic and anti-cancer agents: an update. Korean J Parasitol 2021;59(3):189-225
https://doi.org/10.3347/kjp.2021.59.3.189
Fig. 1Eggs of Trichuris trichiura found in Kato-Katz fecal smears of schoolchildren around Yangon Region, Myanmar showing different sizes during the control project using mass drug administration (2017–2019). (A) Standard-sized (typical) eggs of T. trichiura in a smear from a child. (B) Large- (left) and small-sized (right) eggs of T. trichiura in another child. (C) Standard- (left and middle) and large-sized (right) eggs of T. trichiura in a child. (D) Large- (left, up) and standard-sized (right, down) eggs of T. trichiura, together with fertilized eggs of Ascaris lumbricoides in another child. S Tt, standard-sized T. trichiura egg; L Tt, large-sized T. trichiura egg; Al, A. lumbricoides fertilized egg. Scale bars=90 μm. 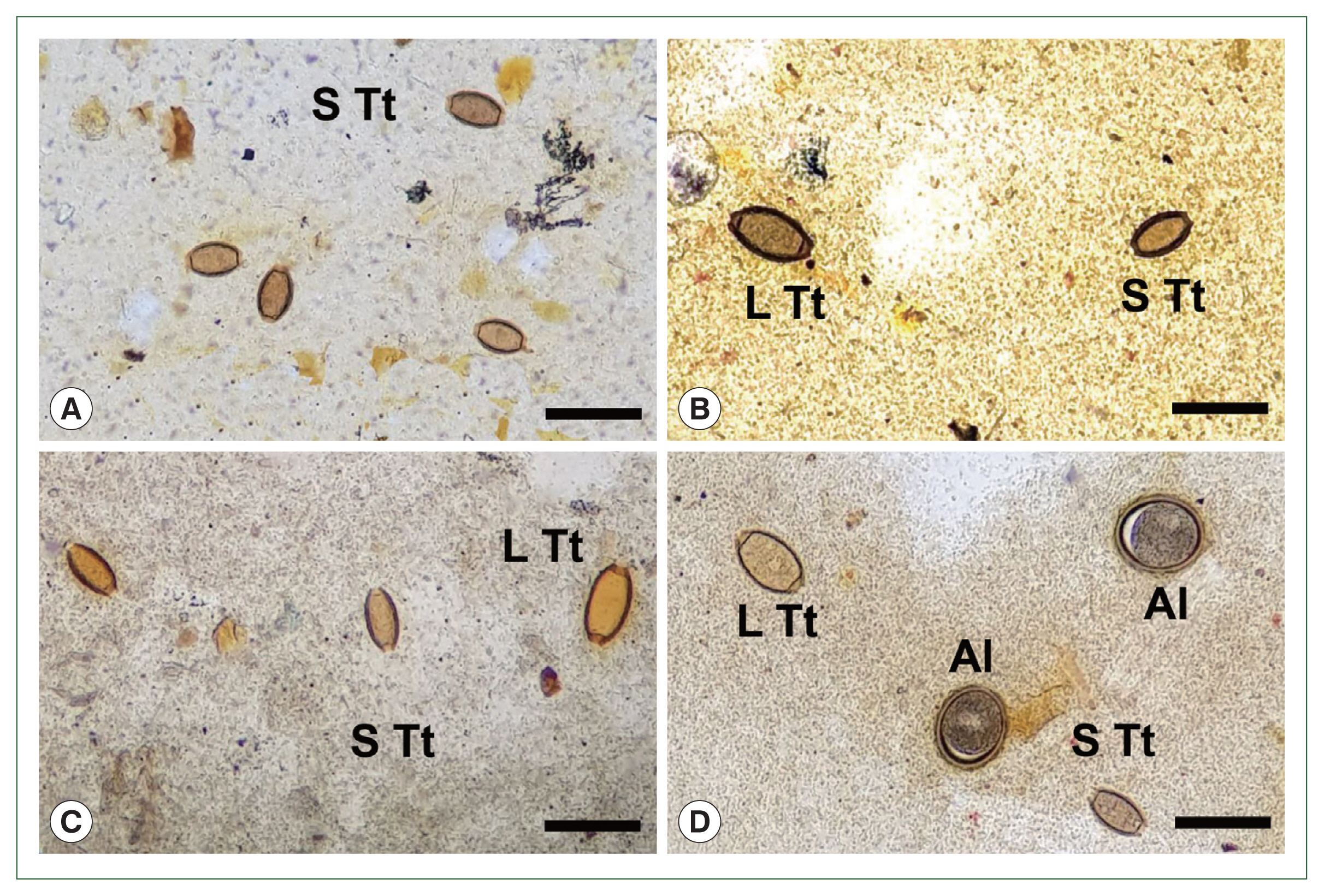
Fig. 2Distribution of the egg sizes of Trichuris spp. in this study (boxes A and B) and in the literature (box C). Box A indicates the size range of standard-sized eggs (normal for T. trichiura) detected in the feces of schoolchildren in the Yangon Region, Myanmar (mean length; 55.2 μm, mean width; 26.1 μm, n=97). Box B indicates the size range of large-sized eggs (mean length; 69.3 μm, mean width; 32.0 μm, n=20). Box C indicates the size ranges of T. vulpis eggs reported previously in the literature (Hall and Sonnenberg 1956 [5]; Dunn et al. 2002 [6]; Areekul et al. 2018 [7]; Márquez-Navarro et al. 2012 [8]). 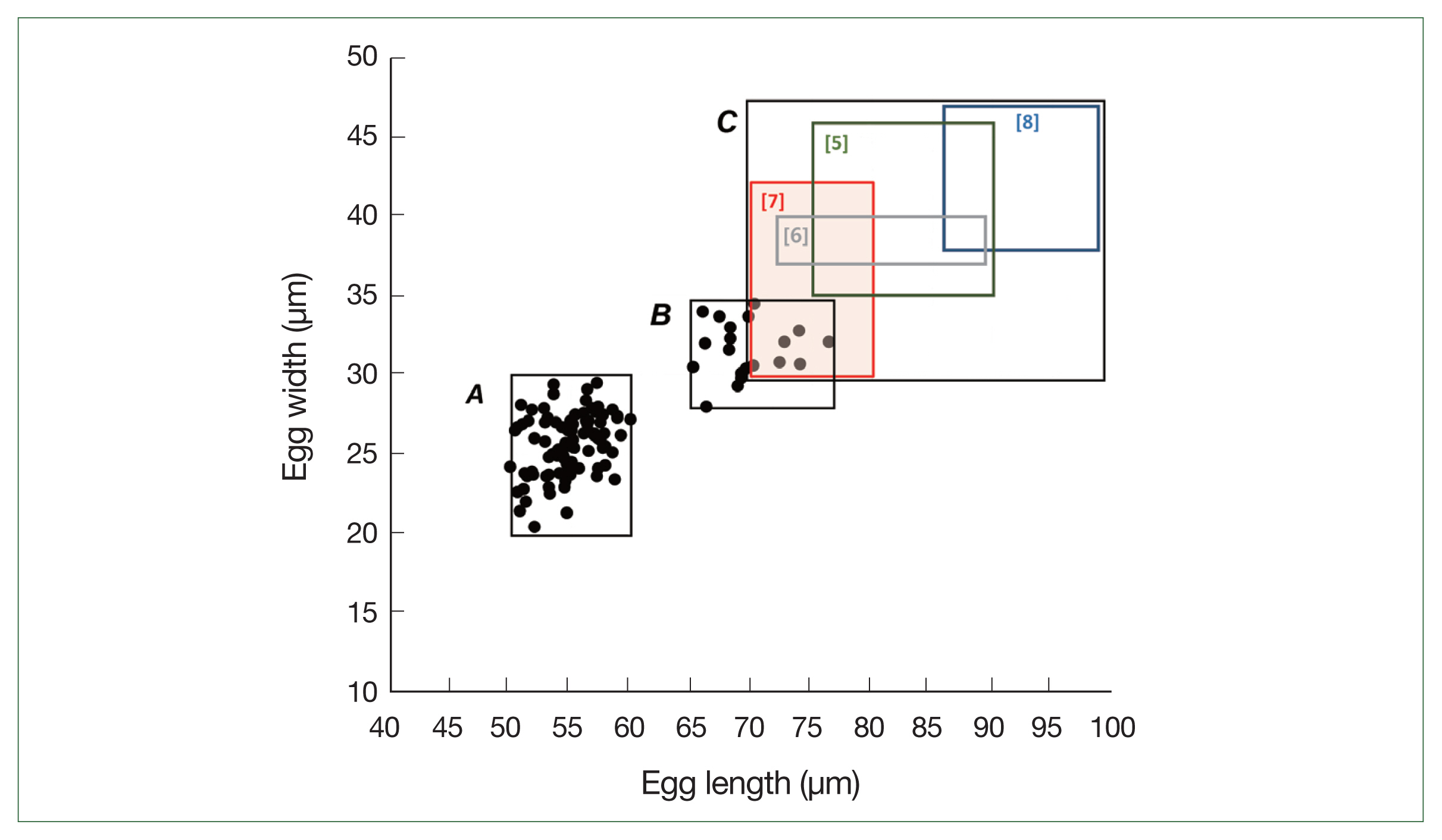
Table 1Measurements and molecular characteristics (18S rDNA sequences) of standard- and large-sized eggs of Trichuris spp. in Kato-Katz fecal smears from schoolchildren in Yangon Region, Myanmar
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||