Abstract
Diagnosis of hydatidosis is based on immunodiagnostic methods along with radiological and ultrasound examinations. The objectives of the present study were to develop a specific and simple antigen-based ELISA method for diagnosis of hydatidosis and compare it with antibody detection method. The subjects in this study included 89 patients in the following groups: surgically confirmed hydatidosis patients (35 cases), control with other parasitic diseases (29 cases), and healthy controls (25 cases). Hyperimmune serum was raised against hydatid cyst fluid in rabbits. Anti-hydatid cyst IgG was purified by affinity chromatography using protein A column and labeled with horseradish peroxidase. Collected sera were assessed for hydatid cyst antigens and antibody by ELISA. Circulating hydatid antigen was found in 9 out of 35 patients with surgically confirmed hydatidosis. A sensitivity of 25.7% and a specificity of 98.0% were calculated for the antigen detection assay. Antibody detection by indirect ELISA, using antigen B, showed that 94.2% of patients (33 cases) have anti-hydatid cyst antibodies in their serum while cross reaction was noted in a few of non-hydatidosis patients. A sensitivity of 94.2% and specificity of 81.6% were found for the antibody detection assay. Findings of this study indicated that antibody detection assay is a sensitive approach for diagnosis of hydatid cyst while antigen detection assay might be a useful approach for assessment of the efficacy of treatment especially after removal of the cyst.
-
Key words: Echinococcus granulosus, hydatidosis, ELISA, diagnosis, antigen detection
INTRODUCTION
Cystic echinococcosis (CE) is a silent cyclozoonotic infection of humans and domestic animals caused by larvae of the cestode
Echinococcus granulosus [
1]. This parasite has a worldwide distribution and is one of the most important zoonotic diseases prevalent in different parts of the world including the Middle East [
1,
2].
The diagnosis of hydatidosis is based on immunodiagnostic methods along with radiological and ultrasound examinations [
3,
4]. A great number of immunological assays have been developed for detection of anti-hydatid cyst antibodies and recently, hydatid antigens in the serum [
5]. These include indirect hemagglutination (IHA), indirect immunofluorescence (IFA), immunoelectrophoresis, counter-current immunoelectrophoresis (CIEP), radioimmunoassay (RIA), and ELISA [
6-
8]. Moreover, enzyme-linked immunoelectrotransfer blots (EITB), enzyme-linked immunoelectrodiffusion assay (ELIEDA), time-resolved fluoroimmunoassay (TR-FLA), and immunoblot assay have been developed for detection of anti-hydatid cyst antibodies [
9-
12]. The main drawback of antibody detection assays is that they cannot readily distinguish between past and present infections and cannot be used for assessment of the efficacy of treatments. Antigen detection assay may circumvent this problem [
13]. It has been shown that hydatid cyst antigen can be detected in the serum or urine of hydatidosis patients. Circulating hydatid antigens are present in the serum only during active infection, and the levels of these antigens continue to decrease after surgical removal of the hydatid cyst or successful chemotherapy [
14]. The detection of circulating antigens rather than antibodies might be very useful in the immunodiagnosis of hydatid disease [
15,
16].
The present study aimed to develop a specific and simple antigen-based ELISA method, using hyperimmune serum raised in rabbits against sheep hydatid cyst fluid antigens, and compare it with the antibody detection method.
MATERIALS AND METHODS
Human sera
A total of 35 sera were collected from pathologically confirmed hydatidosis patients at Shiraz hospitals. Sixty percent of the patients (21 cases) were females and 40% (14 cases) were males. The mean age of the subjects was 37.0 years. To find out any possible cross reactions, sera were also collected from healthy controls (25 cases) and ascariasis (8 samples), strongyloidiasis (8), taeniasis (8), toxoplasmosis (2), and visceral leishmaniasis (3) patients from different parts of Iran.
Hydatid cyst antigen
The hydatid cyst fluid (HCF) was aseptically obtained from the hydatid cysts in sheep collected from Shiraz abattoirs. To remove the protoscolices and large materials, HCF was centrifuged (1,000 g for 30 min). Protein content of the sample was determined by the Bradford protein assay [
17]. The collected antigen was stored at -20℃ until use.
Hyperimmune sera were raised against hydatid cyst fluid in 2 one-year-old male rabbits, each 2-2.5 kg in weight, as described by Shariff and Parija with some modification [
18]. Briefly, HCF was emulsified with an equal volume of Freund's complete adjuvant. Adult rabbits were injected, intramuscularly, with 0.5 ml of this emulsion in all 4 limbs. After 6 wk, they were re-injected, intramuscularly, with 0.5 ml of the same antigen in Freund's incomplete adjuvant in each limb. Serum samples were taken by ear vein bleeding and monitored for the antibodies against HCF by an indirect ELISA.
Affinity chromatography was used to purify IgG from immunized rabbit sera. One gram of dry protein A Sepharose (Sigma, St. Louis, Missouri, USA) was swollen in phosphate buffered saline (PBS) and washed 2 times with 50 ml PBS. The washed Sepharose was packed into a 10-ml syringe with a piece of glass wool and a tap to secure the column. The column was equilibrated by washing with 10 column volumes of PBS, followed by a wash with elution buffer, glycine-HCl, pH 2.8, and once again with 10 column volumes of PBS. Three ml of serum was mixed with an equal volume of PBS and loaded onto the column. PBS was passed through the column until the absorbance of the displaced solution returned to zero. Elution buffer was loaded onto the column and 10 fractions, each 2 ml, were collected in Eppendorf tubes which contained 200 µl of Tris buffer pH 7.8, to neutralize the pH. The absorbance of the eluted samples were measured, peak fractions were pooled together and dialyzed against PBS, overnight at 4℃. Finally, the absorbance of the dialyzed sample was measured and the concentration of IgG was calculated, using the following equation: concentration of IgG (mg/ml) = absorbance (280) × 0.7.
Labeling antibodies with horseradish peroxidase
Conjugation of antibodies was performed as mainly described by Wilson and Nakane [
19]. Briefly, 4 mg of horseradish peroxidase was dissolved in 1 ml of double distilled water (ddH
2O) and mixed with 200 µl of freshly prepared 0.1 M sodium periodate for 20 min at room temperature (RT). The mixture was dialyzed against 1 mM acetate buffer, overnight at 4℃. After dialysis, the pH of mixture was raised to 9.5 by addition of 20 µl of 0.2 M sodium carbonate/bicarbonate buffer. Immediately 8 mg of rabbit anti-hydatid cyst IgG which had been dialyzed against 50 mM sodium carbonate/bicarbonate buffer, pH 9.5, was added to the solution and mixed for 2 hr at RT on a rotator. Then, 100 µl of freshly prepared sodium borohydride (4 mg/ml) was added and the solution was mixed again for 2 hr at 4℃. Finally the solution was dialyzed against PBS overnight and the conjugated antibody was stored at -20℃ in small aliquots until use.
All serum samples along with control samples and samples from ascariasis, strongyloidiasis, taeniasis, toxoplasmosis, and visceral leishmaniasis patients were tested by the capture ELISA system. To perform the capture ELISA, the optimal concentration of rabbit anti-HCF IgG (5 µg/ml) was diluted in coating buffer and 100 µl of solution was placed into a 96-well ELISA plate followed by incubation at 4℃ overnight. The excess antibody was removed by washing the plate 5 times in PBS-Tween 20 (PBST, pH 7.4 containing 0.05% Tween 20). After washing, 100 µl of blocking buffer (3% skimmed milk) was added and the plate was incubated for 1.5 hr at RT. Following the washing steps, sera from surgically confirmed CE patients along with samples from healthy people as negative controls (diluted 1/100 with PBST) was added into the wells and the plate was incubated for another 1.5 hr. After a washing step, 100 µl of horseradish peroxidase-conjugated anti-HCF IgG was added and the plate was incubated for 1 hr at RT. After being washed as before, the plate was incubated with chromogen/substrate (100 µl/well of 4 mg/ml OPD, 0.025% H2O2 in 0.1 M citrate buffer, pH 5.0), and the reaction terminated with 1 mM sulphuric acid after 30 min. The absorbance at 490 nm was monitored with a microplate reader (Bio-TEK- ELX-800). The cut-off point was set as 2SD above the mean of control samples.
Indirect ELISA
Indirect ELISA, using antigen B, was used as described before, for detection of antibodies in sera of CE patients [
7].
RESULTS
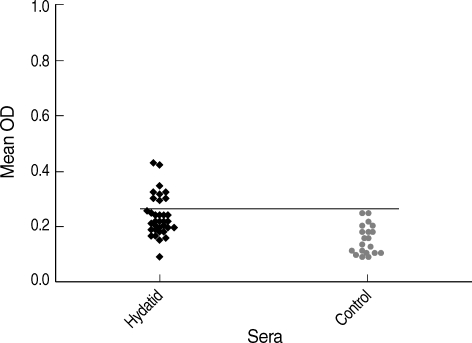
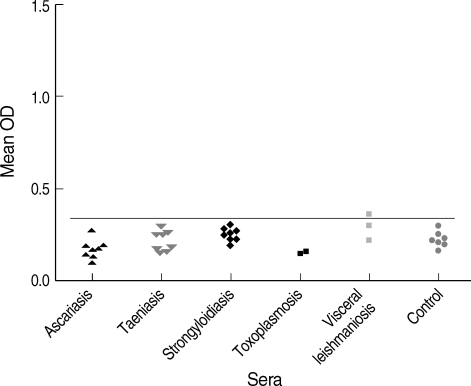
Fig. 1 shows the results of capture ELISA on sera of hydatidosis patients. The hydatid cyst antigen was detected in 9 out of 35 (25.7%) of sera among hydatidosis patients. Apart from a sample from visceral leishmaniasis patient, antigen was not detected in any other patients with parasitic diseases (
Fig. 2). Using these collections of samples, the test had 98.1% specificity (95% confidence interval: 88.8-99.9%) and 25.7% sensitivity (95% confidence interval: 13.1-43.5%) in detecting hydatid cyst antigen in sera of hydatidosis patients. Accordingly, positive and negative predictive values of the system were 90% and 67%, respectively. Antibody detection by indirect ELISA, using antigen B, showed that 94.2% of patients (33 cases) have anti-CE antibodies in their sera while cross reaction was noted in a few of non-CE patients. Therefore, a sensitivity of 94.2% (95% confidence interval: 79.4-99%) and specificity of 81.6% (95% confidence interval: 67.4-90.7%) was calculated for the antibody detection assay.
DISCUSSION
The main problems in the immunodiagnosis of hydatidosis are the often disappointing performances of the available tests and the difficulties associated with the standardization of antigen preparations and techniques [
15,
20,
21]. Most of the serological tests such as ELISA performed on patients' sera for detection of specific antibodies gave rise to variable results of sensitivity and specificity. False negative results in human hydatidosis composes a serious enigma in attaining a conclusive result, as its rate may be 3-5% of hydatid patients and even up to 35-40% in hyper-endemic areas [
15,
20]. The antibody is not raised in some of the hydatidosis patients or the titer is low especially in old persons and infants. Also in cerebral, ocular, and calcified cysts, the antibody titer is low and cannot be easily detected [
22]. On the other hand, the long persistence of anti-
E. granulosus antibodies after surgical removal of the cysts results in unreliable diagnosis of relapse in patients [
23].
Circulating hydatid antigens are present in the serum only during active infection, and the levels of these antigens continue to decrease after surgical removal of the hydatid cyst or successful chemotherapy [
14]. Therefore, some researchers have tried to detect the
E. granulosus antigens in sera of hydatidosis patients especially after hydatid cyst surgery. The general idea is to detect circulating antigens as well as immune complexes and excreted metacestode antigens in sera by different immunological assays. It has been shown that hydatid cyst antigen can be detected in sera of 33-85% of hydatidosis patients [
20,
22]. In a study conducted by Gottstein [
20], hydatid cyst antigen was detected in patients who had not shown anti-hydatid cyst antibodies [
20]. The serum level of hydatid antigen has been found to be up to 270 ng/ml in hydatid cyst patients. Antigen detection assays for diagnosis of hydatidosis resulted in various rates of sensitivity and specificity [
3,
15,
18,
20,
22].
In the present study, the presence of circulating antigens in sera of hydatidosis patients was demonstrated. The low sensitivity of the antigen detection assay in the current study might be related to the fact that most of antigens in sera are immune complexes of antigens and antibodies which cannot be easily detected by serological assays. Moreover, the intact cysts, small cysts, and cysts in privileged sites do not release enough antigens to be detected in the serum. Location of the cysts is an important feature in diagnosis of CE as in our study antigen was detected in up to 46% of patients with liver but not lung or kidney cysts. The reason for the false positivity of a visceral leishmaniasis case in our study is not clear. The high level of antibodies in the visceral leishmaniasis patient might interfere with the antigen detection assay.
When the results of the antigen detection were compared with those of the antibody detection, it was found that 94.2% of patients (33 cases) have anti-CE antibodies in their serum. Although the sensitivity of the antibody detection assay was high, its specificity was rather low since cross reaction was noted in sera of patients with ascariasis, strongyloidiasis, and 1 healthy control. Moreover, the antibody detection assay cannot discriminate between the past and present infections in CE.
Taken together, findings of this study indicated that the antigen detection assay might be a useful method for diagnosis of patients with hydatidosis, although the antibody detection assay has a relatively high sensitivity. Also, the antigen detection assay might be a useful approach for assessment of the efficacy of treatment especially after removal of the cyst.
ACKNOWLEDGEMENTS
The authors would like to thank Mrs. S. Kazemian for her technical assistance, the staff of Nemazee hospital for their cooperation in the sample collection. The technical assistance of Mr. S. Roeeintan is also acknowledged. This study was financially supported by a grant (No: 2809) from office of Vice Chancellor for Research at the Shiraz University of Medical Sciences.
References
Fig. 1Antigen detection in the serum by sandwich ELISA using the hydatid cystic fluid.

Fig. 2Antigen detection in the serum by sandwich ELISA using the hydatid cyst fluid, evaluating possible cross reactions.