Abstract
Differential diagnosis of Taenia asiatica infection from other human taeniases by serology has been tested. An enzyme-linked immunoelectrotransfer blot (EITB) was applied to subjected human sera and tapeworm materials. Thirty-eight proteins reactive to serum IgG were observed between 121 and 10 kDa in adult worms, and more than 22 serum-reactive components between 97 kDa and 21.5 kDa were observed in eggs of T. asiatica. Antigens of adult T. asiatica revealed immunoblot bands between 120 and 21.5 kDa against T. asiatica infected sera. Antigens of adult Taenia saginata revealed 110-100, 66, 58-56, and 46 kDa immunoblot bands against T. asiatica infected sera. Antigens of adult Taenia solium also revealed 99-97, 68-66, and 46 kDa bands against T. asiatica infected sera. The immunoblot band of 21.5 kDa exhibited specificity to T. asiatica.
-
Key words: Taenia asiatica, Taenia saginata, Taenia solium, taeniasis, immunoblot
Taenia solium,
Taenia saginata, and
Taenia asiatica are human-infecting taeniid tapeworms that cause cysticercosis in pigs and cows, and taeniasis in humans. In addition,
T. solium eggs can infect humans, giving rise to neurocysticercosis caused by larval cysticerci developing in the central nervous system.
T. asiatica has been described on the basis of the morphological characteristics of its metacestodes and adult worms, life cycles, host ranges, and a variety of molecular characteristics [
1-
3]. As a result of these examinations,
T. asiatica appears to differ substantially from
T. saginata [
1-
3].
Taeniases remain important public health concerns in regions of Africa, Eastern Europe, and Central and South America. For T. asiatica, however, reports on geographical distribution, epidemiology, pathology, and immunodiagnosis are relatively rare, with the exception of some morphological and genotypic studies. Immunodiagnostic testing by ELISA is one of the most useful methods for epidemiological investigations in endemic areas. ELISA is a highly sensitive diagnostic method. However, differential diagnosis is obviously problematic even though the detection assays developed thus far have increased the sensitivity of taeniases detection. Coproscopical examination based on the morphology of tapeworm proglottids has a low sensitivity and a high technical skill requirement, and human Taenia tapeworm eggs are morphologically indistinguishable.
Coproantigen assays including antigen-capturing ELISA can detect parasite antigens excreted in the stool, and the specificity and sensitivity of antigen-capture ELISA were greater than 99% for human taeniasis [
4-
6]. However, unfortunately, this assay cannot be used to distinguish
T. solium from
T. saginata infections [
4-
6]. To cope with this problem, monoclonal antibodies were developed against defined antigens, and this has improved the sensitivity and specificity of the serological methods utilized for the diagnosis of
T. solium and
T. saginata cysticercosis. Differential diagnosis is of clinical importance for the treatment and control of taeniases in endemic areas.
If serological methods are used in combination with DNA molecular techniques, increased detection sensitivity would be achieved. An assay technique which uses species-specific primers to detect DNA differences between
T. solium and
T. saginata tapeworms has been developed [
7]. More recently, DNA differential diagnosis of taeniasis and cysticercosis by multiplex PCR has been reported [
8]. These techniques depend on the existence of eggs and proglottids in the stool. Although these methods are effective in differential diagnosis, the assays may not be always suitable since eggs and proglottids are only intermittently released into the stool. As immunoserological diagnosis is more practical for studies in aspects of public health and epidemiology, and since no previous works have reported serodiagnosis of human taeniasis caused by
T. asiatica, we designed a differential diagnostic assay using an enzyme-linked immunotransfer blot (EITB) with adult
Taenia tapeworms and eggs as antigens.
Taenia adult worms were collected from individuals infected with
T. solium (China),
T. saginata (Belgium), and
T. asiatica (Korea) following treatment with niclosamide. The parasites were stored at -70℃ in phosphate-buffered saline (pH 7.4) containing protease inhibitors phenylmethylsulfonyl fluoride (PMSF) and leupeptin. A crude antigen preparation of
Taenia adult worms was obtained from gravid proglottids which were homogenized in phosphate buffered saline (pH 7.4) containing protease inhibitors (10 mM PMSF and 2.5 mM leupeptin), and centrifuged for 60 min at 15,000 g at 4℃. After slowly stirring the supernatant for 2 hr, the soluble extract was collected. Protein concentrations were estimated by the method of Bradford [
9].
Serum samples used to assess the specificity and cross-reactivity of antibodies were collected from 51 proven patients having cestode or trematode infections. The samples collected from individuals with only 1 kind of parasitosis were selected on the basis of parasitological examinations by identifying the eggs, larval, or adult worms. T. asiatica (n = 5), T. saginata (n = 3), and T. solium (n = 5) infected sera were obtained from infected Koreans. Adult parasite specimens were recovered from the patients after treatment. The diagnosis of cysticercus (n = 5), sparganum (n = 3), Diphyllobothrium latum (n = 10), and Hymenolepis nana (n = 10) infection was confirmed parasitologically and histologically in individuals aged from 21 to 78 year. Normal human sera were prepared from 46 healthy Korean residents.
SDS-PAGE and immunoblot were performed as previously described [
10]. SDS-PAGE was conducted in 10% gel with the Tris-Tricine buffer system under reducing conditions. Each of the samples contained 70 µg of protein. After electrophoresis, the crude antigens were transferred to a nitrocellulose membrane (Amersham Pharmacia Biotech, Uppsala, Sweden) in semi-dry transfer cells (Bio-Rad, Hercules, California, USA). For the analysis of individual serum samples, the blots were cut into identical 3-5 mm strips. Nitrocellulose strips were incubated with sera diluted to 1 : 100 in Tris buffered saline (100 mM Tris/HCl, 0.85% NaCl, pH 7.4) containing 3% non-fat dry milk and 0.1% Tween-20, for 1 hr at room temperature. Antigen bands that reacted with the human antibodies were visualized with a 1 : 1,000 dilution of peroxidase-conjugated goat anti-human IgG (Jackson Immunoresearch, West Glove, Pennsylvania, USA). The chromogenic substrate was H
2O
2 and 3, 3'-diaminobenzidine. Prestained protein standards were from Amersham Pharmacia Biotech (RPN 756, Little Chalfont, Buckinghamshire, England).
The serum antibody levels in patients with
Taenia tapeworm infections were quantified by micro-ELISA.
Table 1 shows reactivities of
T. asiatica,
T. saginata, and
T. solium crude antigen preparations against serum samples from patients with taeniasis, cysticercosis, sparganosis, and diphyllobothriasis as well as healthy individuals. With antigen concentrations of 5 µl/ml protein on micro-ELISA,
T. asiatica,
T. saginata, and
T. solium antigens were detected in the sera collected from patients infected with these specific parasites, with relative antibody levels in absorbance (abs.) (mean ± SD) of 0.38 ± 0.03, 0.32 ± 0.01, and 0.29 ± 0.01, respectively. Each specific-parasite infected serum cross-reacted with antigens of other
Taenia species. In addition, serum samples from 5 patients with cysticercus infection (abs. 0.26 ± 0.02) and 3 with sparganum infection (abs. 0.47 ± 0.03) showed significant levels of cross-reactivity with the antigens from
Taenia adult worms. Patients' sera from trematode or
Diphyllobothrium infections did not cross-react with
Taenia antigens.
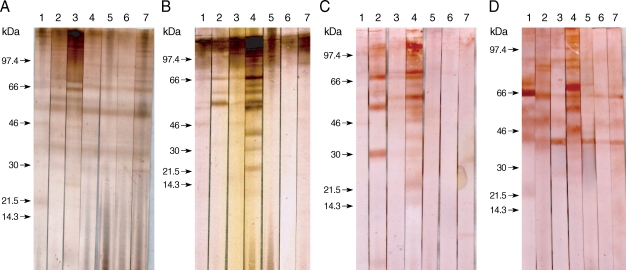
The specific component proteins detected by patient serum IgG were observed by the EITB using SDS-PAGE and a Tris/Tricine discontinuous buffer system with a 10% separating gel. Crude antigens from gravid proglottids of
T. asiatica were separated into more than 30 protein bands, ranging from 10 to > 120 kDa, by SDS-PAGE. Sera collected from 5 patients infected with
T. asiatica exhibited similar patterns of reactivity and detected several antigens, ranging in size from 120 to 21.5 kDa, in the
T. asiatica adult worm antigen preparation (
Fig. 1A). Sera collected from patients with 7 different types of cestode infections exhibited different immunoblot patterns against antigens of
T. asiatica,
T. saginata, and
T. solium adults, as well as
T. asiatica eggs, as shown in
Fig. 1. Patients' serum IgG reacted with many bands between 120 kDa and 21.5 kDa in the adult
T. asiatica crude antigen sample. The 120, 110-100, 98-96, 68-66, 58-56, and 37 kDa bands of
T. asiatica crude antigen cross-reacted with other cestodiases, but the 21.5 kDa band was specific to
T. asiatica (
Fig. 1A, lane 1). However, among those protein bands detected in the sera collected from patients with
T. saginata infections, the 85, 75, 64, and 30 kDa bands were not detected by sera from patients with
T. asiatica infections (
Fig. 1A, lane 2). The serum from a
T. solium-infected patient was found to cross-react with bands at 68, 64, and 25 kDa, which were not detected in the sera from
T. asiatica patients (
Fig. 1A, lane 3).
Adult
T. saginata crude antigens blotted with sera from patients with
T. saginata infections detected 110-100, 86, and 67 kDa bands as the major antigens (
Fig. 1B, lane 2). Sera from
T. asiatica-infected patients detected 110-100, 66, 58-56, and 46 kDa bands (
Fig. 1B, lane 1). Proteins at 110-100, 66, 58, and 46 kDa were detected in the sera of
T. solium infected patients (
Fig. 1B, lane 3). In contrast,
T. solium antigen bands at 98, 69-67, 60, and 58 kDa reacted to the sera from
T. solium-infected patients (
Fig. 1C, lane 3), and bands at 99-97, 68-66, and 46 kDa reacted against the serum of a
T. asiatica-infected individual, although the cross-reactivity was faint (
Fig. 1C, lane 1). The serum collected from a
T. saginata-infected individual cross-reacted with
T. solium bands at 69-67, 58-56, and 30-28 kDa (
Fig. 1C, lane 2).
The egg antigens of
T. asiatica showed more than 15 protein bands by SDS-PAGE, ranging from 8 to 120 kDa. The 21.5 kDa band was the major antigenic protein detected in the sera of
T. asiatica-infected individuals (
Fig. 1D, lane 1). Bands at 85, 83, 65, 50, and 45 kDa were detected in the sera from
T. saginata-infected patients (
Fig. 1D, lane 2) and bands at 99, 90-88, 65, 50, and 45 kDa cross-reacted with sera from
T. solium-infected individuals (
Fig. 1D, lane 3).
T. asiatica has been described fairly recently, and is distributed throughout Taiwan, Korea, China, Indonesia, the Philippines, Vietnam and Thailand [
1,
11-
15].
T. asiatica,
T. saginata, and
T. solium coexist in China, Korea, Vietnam, and Thailand [
13-
15]. It is crucial to determine which individuals are infected by
T. solium, due to the possibility of cysticercosis in humans. Early detection and adequate treatment of taeniasis is not only relevant to prevention of cysticercosis, but also critical for reliable epidemiological information for effective control of taeniasis and cysticercosis.
T. saginata and
T. asiatica are often confused because of their morphological similarities. The most accurate methods for species identification are predicated on molecular and immunological diagnostics, coupled with comparative morphology [
16].
A previous study of antigenic components of
T. saginata and
T. solium reported approximately 20 to 23 antigens as a result of immunoprecipitation [
7]. In the present study, SDS-PAGE yielded more than 30 protein bands in crude antigen preparations of adult
T. asiatica, and more than 15 of these bands were detected in the sera of
T. asiatica-infected individuals.
T. asiatica and
T. saginata are sister species and thus expected to share some common antigenic determinants.
Epidemiologically,
T. asiatica is prevalent in Korea, where it co-exists with
T. solium. These parasites both utilize the pig as an intermediate host. The larval stages of
T. asiatica (= Cysticercus viscerotropica) in the pig exhibit a marked liver tropism which causes hepatic cysticercosis in the intermediate host [
2], as well as in experimental intermediate hosts such as calves, goats, and monkeys [
17]. If C. viscerotropica follows the same developmental course as seen in intermediate host animals, it might cause hepatic cysticercosis in the human host also. The possible clinical symptoms of C. viscerotropica infection in man, if any, are more likely to be similar with the initial stages of cystic hydatidosis rather than the classical cysticersosis or neurocysticercosis associated with
T. solium infection, although human infectivity by the eggs of
T. asiatica remains a matter of some controversy [
18]. The clinical importance of infections by
T. asiatica, as well as the potential for cysticercosis attributable to
T. asiatica in humans, requires further study. In our study, we did not address differential immunodiagnosis between the larval and adult stages; however, the primary target antigen bands of adult
T. asiatica were readily detected by immunoblotting. Finally, our results clearly indicate that
T. asiatica and
T. saginata can be differentiated to the species level by immunoblotting, and this assay system should prove applicable to serological and molecular epidemiological investigations into taeniid parasite infections.
ACKNOWLEDGEMENTS
This work was supported by a research grant from Chungbuk National University in 2007. Parasite materials used in this study were provided by the Parasite Resource Bank of Korea National Research Resource Center (R21-2005-000-10007-0), Republic of Korea.
References
Fig. 1Immunoblot patterns of human sera against the adult and egg antigens of Taenia tapeworms. The Taenia proteins were separated by SDS-PAGE on nitrocellulose membranes, and then blotted with serum samples. The blotted proteins were from T. asiatica (A), T. saginata (B), T. solium (C), and egg antigens of T. asiatica (D). Blot strips were probed with sera from patients showing taeniasis asiatica (lane 1), taeniasis saginata (lane 2), taeniasis solium (lane 3), cysticercosis (lane 4), sparganosis (lane 5), diphyllobothriasis (lane 6), and hymenolepiasis (lane 7). Arrows indicate the 21.5 kDa band specific to T. asiatica.
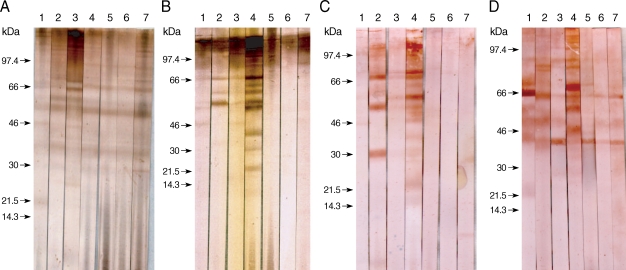
Table 1.Mean and standard deviation of antibody levels in absorbance as measured by micro-ELISA in patients’ sera of cestodiasis
Table 1.
|
Patient category |
No. of cases tested |
Absorbance (mean±SD) of positive cases to the antigensa of
|
|
T. asiatica
|
T. saginata
|
T. solium
|
|
Taeniasis asiatica |
5 |
0.38 ± 0.03 |
0.28 ± 0.02 |
0.35 ± 0.03 |
|
Taeniasis saginata |
3 |
0.31 ± 0.04 |
0.32 ± 0.01 |
0.27 ± 0.02 |
|
Taeniasis solium |
5 |
0.25 ± 0.03 |
0.32 ± 0.02 |
0.29 ± 0.01 |
|
Cysticercosis |
5 |
0.26 ± 0.02 |
0.27 ± 0.02 |
0.32 ± 0.01 |
|
Sparganosis |
3 |
0.47 ± 0.03 |
0.47 ± 0.06 |
0.46 ± 0.03 |
|
Diphyllobothriasis |
10 |
0.10 ± 0.01 |
0.10 ± 0.01 |
0.10 ± 0.01 |
|
Paragonimiasis |
10 |
0.10 ± 0.01 |
0.10 ± 0.01 |
0.10 ± 0.01 |
|
Clonorchiasis |
10 |
0.06 ± 0.005 |
0.05 ± 0.005 |
0.05 ± 0.005 |
|
Normal control |
46 |
0.10 ± 0.006 |
0.10 ± 0.005 |
0.10 ± 0.006 |