Abstract
Cockroaches have been recognized as a major cause of asthma. Bla g 4 is one of the most important German cockroach allergens. The aim of this study is to investigate IgE reactivity to the recombinant Bla g 4 (rBla g 4) in the sera of allergic patients and identify linear IgE binding epitope. For protein expression, full-length Bla g 4 (EF202172) was divided into 5 overlapping peptide fragments (E1: aa 1-100, E2: aa 34-77, E3: aa 74-117, E4: aa 114-156, and E5: aa 153-182). The full-length and 5 peptide fragments of Bla g 4 was generated by PCR and over-expressed in E. coli BL21 (DE3). The IgE binding reactivities of the full-length and peptide fragments were measured by ELISA using 32 serum samples of cockroach allergy. The sera of 8 patients (25%) reacted with rBla g 4. Four sera (100%) showed IgE-binding reactivity to full-length and peptide fragment 4, and 2 sera (50%) reacted with peptide fragment 2. One (20%) serum reacted with peptide fragment 3. The results of ELISA using overlapping recombinant fragments indicated that the epitope region was located at amino acid sequences 34-73 and 78-113, and major IgE epitope of Bla g 4 was located at amino acid sequences 118-152 of C-terminal. B-cell epitope analysis of German cockroach allergen Bla g 4 could contribute to the strategic development of more specific and potentially efficacious immunotherapy.
-
Key words: Blattella germanica, Allergen, B-cell epitope, IgE, lipocalin
INTRODUCTION
Several German cockroach allergens, which stimulate IgE production and cause IgE-mediated diseases, have been identified. Bla g 4 is a species-specific allergen produced by
Blatella germanica [
1] and expressed in a sex-specific manner in the reproductive system of adult male German cockroaches. It is associated with the spermatophore, transferred to the female during copulation and developmentally regulated by juvenile hormones [
2]. Although the sequence identity between Bla g 4 and lipocalins is low (19-24%), it contains the structurally conserved regions (SCR) of lipocalin [
3]. Structural homology between other allergenic lipocalins (Rat n 1, Can f 1, Can f 2, Bos d 2, Bos d 5, Equ c 1, and Equ c 2) is known to be high [
4]. The structural model shows it to be cup-shaped with 8 strands and an anti-parallel β, barrel shape [
5]. Higher expression yields of Bla g 4 can be obtained in
Pichia pastoris than in
Escherichia coli [
6]. Accordingly, Bla g 4 warrants further investigation for its role in causing inflammation in patients with asthma.
Recombinant allergens for immunotherapy may be used to alter immune responses or to reduce the allergenic activity of other allergens. Genetically modified recombinant allergens provide several advantages [
7]. These molecules preserve the repertoire of allergen-specific T-cell epitopes and could inhibit the binding of allergic patients' IgE antibodies to allergens represented by the mutagenesis of B-cell epitopes [
8]. Specially, hypoallergens with reduced allergenic activity could be obtained by site-directed mutagenesis when the information on IgE binding epitopes is available. These studies are useful for designing of specific immunotherapy. Previously, we have investigated the sequence polymorphisms of Bla g 4 [
9]. Furthermore, the sequence diversity of Bla g 4 was found to be produced by the post-transcriptional modification [
10].
Although Bla g 4 is a major allergen, IgE binding epitopes have not been investigated. We are thus compelled to examine their allergenicity of recombinant Bla g 4 fragments.
MATERIALS AND METHODS
Patient sera
A total of 48 sera was included in this study. Thirty-two cockroach-allergic patients and 16 healthy individuals without cockroach allergies were recruited, and their sera were studied. The allergic patients (n = 32, ages ranging from 7 to 58 year) attended the Allergy Clinic of Severance Hospital, Yonsei University College of Medicine, Seoul, Korea. All sera were confirmed for the presence of IgE antibodies > 0.7 kU/L against
B. germanica using the Uni-CAP system (Amersham Pharmacia Biotech, Little Chalfont, UK).
Table 1 shows the characteristics of the 32 allergic patients.
Colonies of German cockroaches, B. germanica, have been maintained in the insect rearing facility of the Korea National Arthropods of Medical Importance Resource Bank, Department of Environmental Medical Biology, Yonsei University College of Medicine, Seoul, Korea. Mouse food was used as a diet. Cockroaches were collected after anesthetized with carbon dioxide gas and kept at -70℃ until use.
Molecular cloning of Bla g 4 cDNA
Total RNA was isolated from 6 adult
B. germanica males using the Trizol reagent (Invitrogen, Carsbad, California, USA) according to manufacturer's instructions. A cDNA encoding Bla g 4 was obtained by reverse transcriptase-PCR (RT-PCR) as described previously [
9]. Primer sequences used for the PCR are as follows: Bg4F (5'-GCAGTTTTGGCACTATGTGC-3' and Bg4R (5'CTTAGTGACATGTGGAGTG-3'). PCR was performed with 35 cycles of 30 sec at 94℃, 30 sec at 50℃, and 1 min at 72℃ in a volume of 50 µL. An initial 5-min incubation at 95℃ was performed, along with a final extension of 9 min at 72℃. The PCR amplified products were ligated into the pGEM-T Easy vector (Promega, Madison, Wisconsin, USA) and transformed into competent
E. coli JM109.
To express the recombinant Bla g 4 in
E. coli, the full-length Bla g 4 cDNA was amplified from pGEM-T Easy-Bla g 4. Bla g 4 (EF202172) [
9] was divided into 5 overlapping peptide fragments (E1: aa 1-100, E2: aa 34-77, E3: aa 74-117, E4: aa 114-156, and E5: aa 153-182) (
Fig. 1). The specific primers used in PCR are listed in
Table 2. PCR was carried out with an annealing temperature of 52℃. The amplified PCR products were ligated into pET-28b (Novagen, Madison, Wisconsin, USA) using
BamHI and
XhoI restriction sites and transformed into
E. coli BL21 (DE3).
For protein expression, the full-length and peptide fragments of Bla g 4 were over-expressed in E. coli BL21 (DE3). A single colony from the positive clones was inoculated into 10 mL of Luria Bertani broth, and cultures were grown overnight at 37℃ with shaking. After 16 hr, the culture was further diluted into 1 L and cultured at 37℃ with vigorous shaking (210 rpm) until the OD600 reached 0.6, when isopropyl-1-thio-β-galactopyranoside (IPTG) was added to a final concentration of 1 mM to induce expression. After 4 hr, the cells were harvested by centrifugation at 3,000 g for 20 min. The pellets of the full-length and peptide fragments (E1, E2, and E4) were resuspended in 20 mM Tris, 500 mM NaCl, 5 mM imidazole, and 6 M urea, pH 7.9, and sonicated for 6 × 10 sec with 10 sec pause at 250 W. The pellets expressing peptide fragments (E3 and E5) were resuspended in 10 mM imidazole, 300 mM NaCl, 50 mM NaH2PO4 at pH 8.0 and lysed using a sonicator. Recombinant proteins were purified by using nickel-nitrilotriacetic acid resin (QIAGEN, Valencia, California, USA) according to the manufacturer's instructions under native conditions or denaturing conditions. Purified recombinant proteins were separated on 18% polyacrylamide gels containing sodium dodecyl sulfate (SDS) under denaturing conditions and visualized by Coomassie brilliant blue.
IgE binding reactivity and IgE epitope analysis of recombinant Bla g 4
Purified recombinant Bla g 4 was diluted to 10 µg/ml in a coating buffer (0.1 M sodium carbonate, pH 9.6). Each well of a 96-well microplate was coated with 100 µl of the recombinant protein and incubated at 4℃ overnight, followed by washing with phosphate-buffered saline (pH 7.4) containing 0.05% Tween 20 (PBST). For the blocking, 200 µl of blocking solution (3% w/v skim milk in PBS) was added individually and the plate was further incubated at room temperature (RT) for 1 hr. Human serum samples (1 : 4 dilutions) were then added and incubated for 2 hr. The wells were washed as previously described. Fifty microliters of biotinylated goat anti-human IgE (1 : 1,000 dilution) (Vector, Burlingame, California, USA) were added to each well and incubated for 1 hr at RT. After washing with PBST, 50 µl of streptavidin-peroxidase (Sigma, St. Louis, Missouri, USA) diluted 1 : 1,000 in the diluent buffer was added to each well and incubated for 30 min. The color was developed using 3,3',5,5'-tetramethyl-benzidine (TMB, Kirkegaard & Perry Laboratories, Gaithersburg, Maryland, USA) as a substrate solution. The plate was kept in the dark for 20 min and the reaction stopped by addition of 100 µl of 0.5 M H2SO4. The absorbance was determined at 450 nm with the automatic microplate reader (TECAN, Salzburg, Austria). The mean absorbance level plus 2 standard deviations of 16 healthy controls was used as a cut-off value.
IgE binding reactivities of the peptide fragments were measured by ELISA using 4 sera samples obtained from recombinant Bla g 4 positive sera. Purified recombinant peptide fragments (10 µg/ml) in a coating buffer (0.1 M sodium carbonate, pH 9.6) were coated into each well of the microtiter plate and ELISA was performed as described above. The cut-off value was the absorbance level plus 2 standard deviations of 4 healthy controls. Each determination was done in duplicate.
RESULTS
Expression of recombinant Bla g 4 and peptide fragments
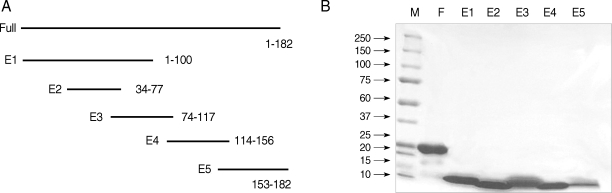
Full-length and peptide fragments containing
BamHI-
XhoI restriction sites were subcloned into the pET-28b expression vector. The recombinant proteins were expressed as N-terminal fusion proteins with 6 histidine residues. The full-length and fragments 1, 2, and 4 were obtained from an insoluble fraction, while fragments 3 and 5 were obtained from a soluble fraction (
Fig. 1A). The protein yield was 2.07 mg/L as measured by the Bradford assay (Bio-rad, Hercules, California, USA). The full-length protein purified by Ni-nitrilotriacetic acid-agarose migrated as a single band with a molecular weight of about 24 kDa. Peptide fragments migrated at molecular weights higher than their predicted sizes on a 5-20% gradient SDS gel (Invitrogen) (
Fig. 1B).
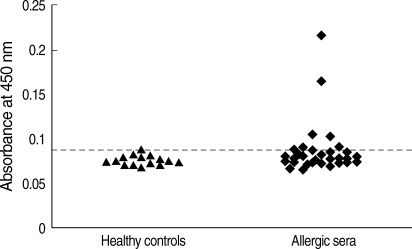
The purified recombinant Bla g 4 was tested for its reactivity with IgE antibodies by ELISA in 32 sera from German cockroach-sensitized patients. Among the 32 sera tested, 25% exhibited IgE reactivity to recombinant Bla g 4 (
Fig. 2). Healthy human sera used as the negative control did not show any IgE reactivity.
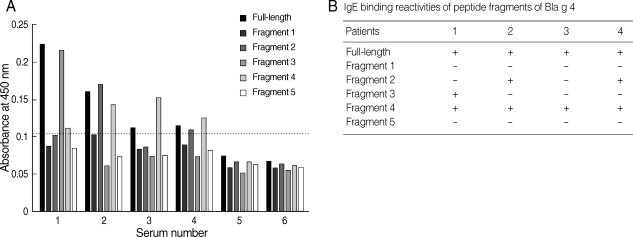
For the determination of IgE epitopes, Bla g 4-derived peptides were expressed as 5 overlapping recombinant peptides E1, E2, E3, E4, and E5. Four sera showing high level absorbances to recombinant Bla g 4 were selected for IgE epitope analysis. The IgE epitope study from Bla g 4-sensitized sera was performed by ELISA and the results were summarized (
Fig. 3). The results showed that IgE-binding sites were heterogeneously located among the different sera (
Fig. 3A). IgE antibodies from patients 2 and 4 were able to recognize fragments 2 and 4. However, fragment 3 was recognized only by IgE antibodies of patient 1. All 4 sera recognized fragment 4. These results indicated that there were at least 3 IgE-binding antigenic determinants.
DISCUSSION
The IgE-binding reactivity of recombinant Bla g 4 was assessed by ELISA, and 25% of German cockroach-sensitized Korean subjects were found to have a positive IgE reactivity to recombinant Bla g 4. This prevalence was significantly lower than the 40-60% previously reported by Arruda et al. [
1]. Bla g 4 has been defined as the major allergen in allergic patients, but these results are in agreement with the results supported by Satinover et al. (prevalence 17.4%, 7/34) [
11] and Slater et al. (prevalence 20.6%, 7/34) [
12]. The discrepancies in the IgE-binding reactivity in the results obtained could be explained by 2 assumptions. One is to start with minor modifications of the complete molecule by amino acid substitution. If the modifications cause changes in the 3-dimensional structure, they could also be expected to affect the IgE binding. Intramolecular disulfide bridges are a characteristic of lipocalins [
13]. Disruption of similar bridges in mite allergens has been shown to interfere with the IgE binding [
14-
17]. Second, the presence of multiple Bla g 4 isoforms in cockroach may influence the allergenicity. The presence of multiple isoforms for other major allergens has also been reported [
18]. The isoforms of Birch pollen allergen Bet v 1 and mite allergen Der p 2, which have 10 and 8 isoforms respectively, were reported to differ not only in the extent of IgE-binding activity but also in T-cell activation [
18,
19].
To determine the IgE-binding epitope, it is necessary to assess the results of the immunological studies with reference to the molecular characteristics. Especially, B-cell epitopes are essential both for the localization of the IgE-binding regions and for the comparative structural analyses of lipocalin allergens [
20]. All the sera tested in this study showed different patterns of IgE-reactivity. Recently, 3 mutants of Bla g 4 at Arg at 24th amino acid, Arg at 26th amino acid, and Lys at 75th amino acid, showed significant reduction in IgE binding in 2 of 24 (8%) sera tested as compared with wild type protein [
21]. This report also implies the heterogeneous IgE binding of Bla g 4. The results in the present study showed that although human IgE antibodies reacted with antigenic determinants distributed throughout the whole molecule, a major IgE-binding region was located at residues 118 to 152, close to the C-terminus of Bla g 4. The B-cell epitopes of Bos d 5 have been studied mostly using protein fragments, where residues 41 to 60, 102 to 124, and 149 to 162 were identified as the major epitopes [
22], a somewhat similar result in Bla g 4 (
Fig. 4). Therefore, comparison between other allergenic lipocalins needs to be done to determine whether these epitopes are conserved in other lipocalin allergens. Heterogeneity in IgE responses to Bla g 4 in individual patients was also observed, which correlated with the finding that the most allergens have multiple IgE-binding sites which is at least partly the result of the polyclonal nature of the immune response to protein allergens [
23-
25].
In summary, the predominant variants and allergenicity of the allergen in different regions can be different. We investigated the predominant variant and linear IgE binding epitope of Bla g 4 because of T-cell epitopes of few lipocalin allergens have been characterized but information of B-cell epitopes have almost never been known.
Analysis of B-cell epitopes may provide new information for manipulation of allergens and production of safer forms of specific immunotherapy with reduced allergenic molecules.
ACKNOWLEDGEMENTS
This work was supported by the Korea Science and Engineering Foundation (KOSEF) grant funded by the Korea government (MOST) (No. R01-2006-000-10997-0).
References
Fig. 1(A) Schematic representation of Bla g 4 fragments for epitope analysis. (B) SDS-PAGE of full-length and peptide fragments. Proteins run on 5-20% gradient SDS-PAGE gel and stained with Coomassie brilliant blue. M, Molecular weight markers in kDa; F, full-length; E1, 1-100 amino acid; E2, 34-77; E3, 74-117; E4, 114-156; and E5, 153-182.
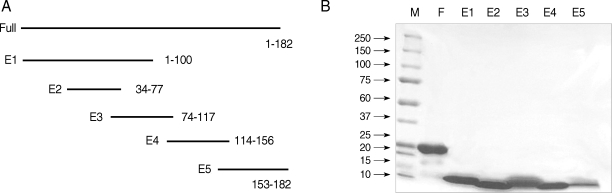
Fig. 2IgE reactivity of human sera against recombinant Bla g 4. The horizontal line indicates the cut-off value. ▴, sera from healthy controls; ♦, sera from German cockroach-sensitized subjects (Uni-CAP > 0.7 kU/L).
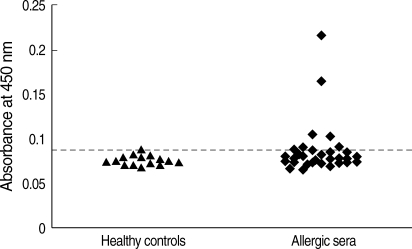
Fig. 3(A) Binding profiles of IgE antibodies to recombinant Bla g 4 and relevant recombinant proteins by ELISA. 1-4, allergic sera; 5, pooled sera from healthy controls; 6, bovine serum albumin. The horizontal line indicates the cut-off value. (B) IgE-binding reactivities of peptide fragments of German cockroach allergen, Bla g 4.
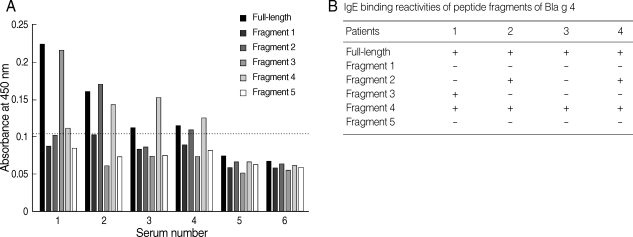
Fig. 4Sequence alignment of allergenic lipocalins: Bla g 4 (Blattella germanica, EF202172), Bos d 5 (Bos domesticus, X14712), Bos d 2 (B. domesticus, L42867), Equ c 1 (Equus caballus, U70823), Can f 1 (Canis familiaris, AF027177), Can f 2 (C. familiaris, AF027178), Mus m 1 (Mus musculus, X03208), Rat n 1 (Rattus norvegicus, M26835). *, fully conserved residues; : , strongly conserved residues; ·, weakly conserved residues. The shaded areas indicate the IgE-binding regions.

Table 1.IgE reactivity of human sera against recombinant Bla g 4
Table 1.
|
Serum sample |
Sexa
|
Age (year) |
Diagnosis |
OD450b
|
|
P1 |
M |
43 |
Allergic asthma |
0.0795 |
|
P2 |
F |
52 |
Dermatofibroma |
0.0855 |
|
P3 |
F |
45 |
Allergic rhinitis |
0.0730 |
|
P4 |
F |
57 |
Allergic asthma |
0.0725 |
|
P5 |
F |
46 |
Allergic rhinitis |
0.0745 |
|
P6 |
M |
49 |
Allergic asthma |
0.0770 |
|
P7 |
F |
22 |
Atopic dermatitis |
0.0785 |
|
P8 |
M |
35 |
Allergic rhinitis |
0.0740 |
|
P9 |
M |
49 |
Allergic asthma |
0.0890c
|
|
P10 |
M |
48 |
Allergic asthma |
0.0865
|
|
P11 |
M |
11 |
Atopic dermatitis |
0.2155
|
|
P12 |
F |
45 |
Allergic asthma |
0.0900
|
|
P13 |
M |
26 |
Atopic dermatitis |
0.0790 |
|
P14 |
M |
45 |
Allergic rhinitis |
0.0910
|
|
P15 |
M |
56 |
Allergic asthma |
0.0860 |
|
P16 |
M |
47 |
Atopic dermatitis |
0.1635
|
|
P17 |
F |
37 |
Allergic asthma |
0.0800 |
|
P18 |
F |
58 |
Allergic asthma |
0.0795 |
|
P19 |
M |
52 |
Allergic asthma |
0.0725 |
|
P20 |
M |
38 |
Allergic rhinitis |
0.0765 |
|
P21 |
M |
26 |
Allergic asthma |
0.0725 |
|
P22 |
M |
33 |
Allergic rhinitis |
0.0835 |
|
P23 |
F |
17 |
Atopic dermatitis |
0.0825 |
|
P24 |
F |
36 |
Allergic asthma |
0.0730 |
|
P25 |
M |
30 |
Allergic rhinitis |
0.0730 |
|
P26 |
M |
7 |
Atopic dermatitis |
0.0810 |
|
P27 |
M |
20 |
Allergic asthma |
0.0725 |
|
P28 |
M |
24 |
Allergic asthma |
0.1030
|
|
P29 |
M |
41 |
Allergic asthma |
0.0680 |
|
P30 |
M |
14 |
Atopic dermatitis |
0.0690 |
|
P31 |
F |
15 |
Atopic dermatitis |
0.1045
|
|
P32 |
M |
53 |
Allergic rhinitis |
0.0680 |
Table 2.Sequences of oligonucleotides used for the production of fragments of Bla g 4
Table 2.
|
Oligonucleotides |
Sequencesa
|
|
Bg4 -E1F |
5′-GGATCCGGCAGTTTTGGCACTA-3′ |
|
Bg4 -E1R |
5′-CTCGAGGTGCTGTATCATTTCT-3′ |
|
Bg4 -E2F |
5′-GGATCCGTGGATTATTGCAGCC-3′ |
|
Bg4 -E2R |
5′-CTCGAGTCTTATAGCAGTCTTA-3′ |
|
Bg4 -E3F |
5′-GGATCCGGCTATAAGAGGACGA-3′ |
|
Bg4 -E3R |
5′-CTCGAGGCCTTCCACAATTGCG-3′ |
|
Bg4 -E4F |
5′-GGATCCAGTGGAAGGCTGTCCC-3′ |
|
Bg4 -E4R |
5′-CTCGAGCACCTGATCCAAAGTG-3′ |
|
Bg4 -E5F |
5′-GGATCCGGATCAGGTGAATCAA-3′ |
|
Bg4 -E5R |
5′-CTCGAGTTAGTTACATGTGGAG-3′ |